
Purification of Recombinant Proteins and Antibodies
Leinco Technologies specializes in purifying antibodies from various sources, including serum, ascites, and culture supernatants. Our robust, multi-step purification processes ensure high-purity antibodies with low endotoxin levels, ideal for in vivo and other critical applications.
Partner with our experienced team for tailored purification solutions that optimize your antibody research. Whether you’re working with small-scale laboratory samples or large-scale commercial batches, we have the expertise and capabilities to meet your needs.
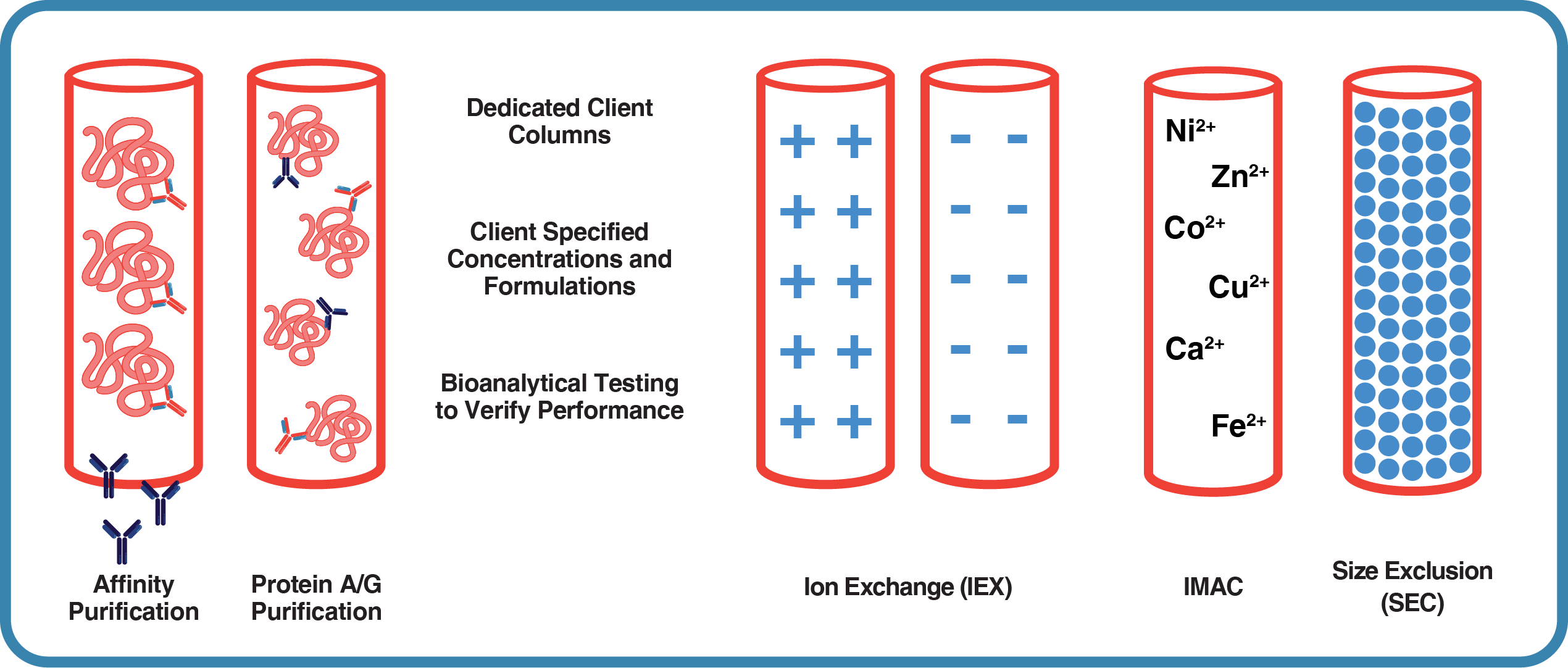
Our Extensive Purification Capabilities Include:
- Custom Affinity Chromatography: Tailored purification using target-specific peptides or recombinant proteins for optimal results.
- Protein A & G Affinity Chromatography: Efficient purification of immunoglobulins based on their Fc region binding properties.
- Aseptic/Low Endotoxin Purification: Specialized purification for in vivo and other sensitive applications requiring low endotoxin levels.
- Ammonium Sulfate Precipitation: A cost-effective method for initial protein concentration and partial purification.
- IgM Affinity Purification: Targeted purification of IgM antibodies using specific affinity ligands.
- Ion-Exchange Chromatography: Versatile purification based on protein charge, offering both anion and cation exchange options.
Trust Leinco Technologies for comprehensive, high-quality protein and antibody purification services tailored to your specific need!
Available Bio-analytical Testing Includes:
- SDS-PAGE Analysis for Purity
- FPLC Analysis for Purity and % Aggregates
- Isoelectric Focusing (IEF) for Identity
- Concentration Determination
- Protein A / Antibody Ratio
- Sterility
- Mycoplasma Testing
- Endotoxin Testing
- Biological Activity
- Conductivity
- pH Determination
- Freeze-Thaw Recovery
Quality Assurance Documentation
The antibody and protein purification process is performed using standard operating procedures (SOPs) and cGMP compliant batch records. Upon completion of a recombinant protein purification batch, a master production file is archived and bio-analytical lot release testing records are provided to clients.


