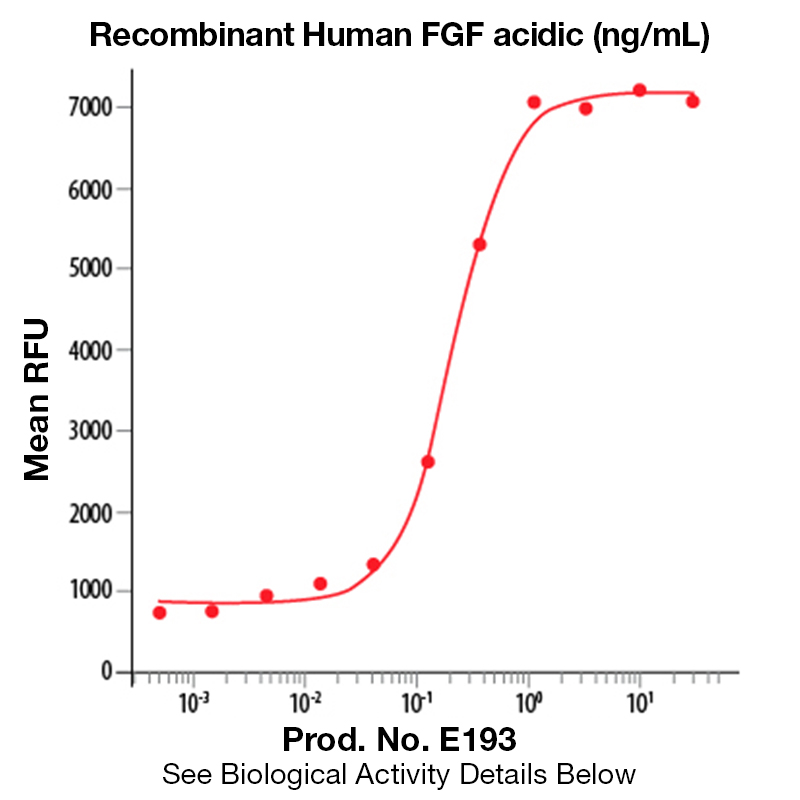
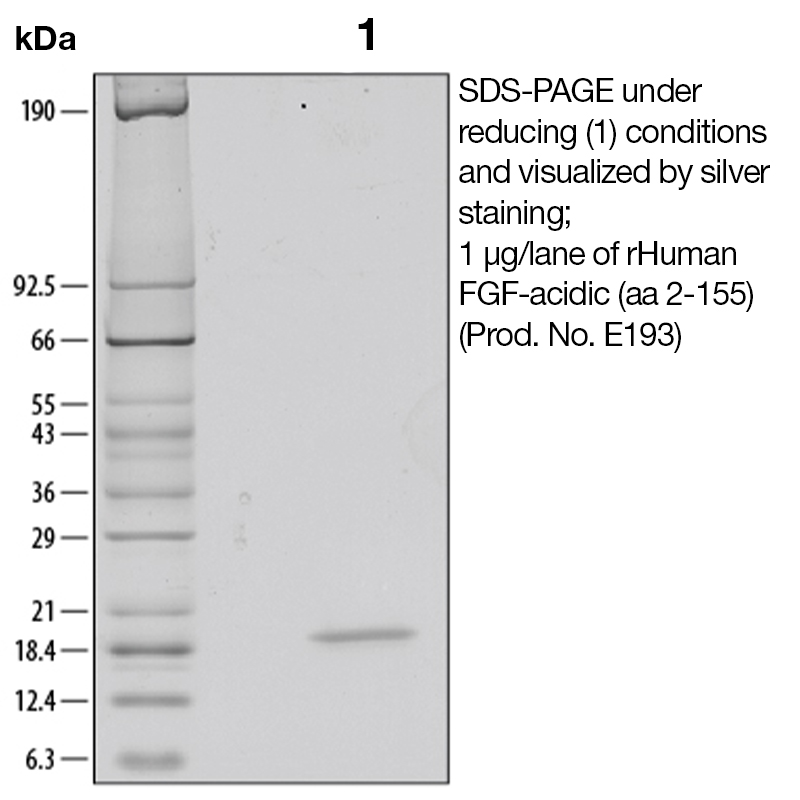
Recombinant Human FGF-Acidic (aa 2-155)
Recombinant Human FGF-Acidic (aa 2-155)
Product No.: E193
- -
- -
Alternate Names Fibroblast Growth Factor-Acidic, FGF-1, ECGF, HBGF-1, AFGF, ECGF-Beta, ECGFA, ECGFB, FGF-Alpha, FGFA, GLIO703 Product Type Recombinant Protein Expression Host E. coli Cells Species Human |
Data
- -
- -
BackgroundAcidic fibroblast growth factor (aFGF), also known as FGF-1, ECGF and HBGF-1, is a non-glycosylated heparin binding growth factor and member of the FGF family of mitogenic peptides. It is involved in several important physiological and pathological processes, such as embryonic development, morphogenesis, angiogenesis, wound healing and atheromatosis (1). aFGF is expressed in the brain, kidney, retina, smooth muscle cells, bone matrix, osteoblasts, astrocytes and endothelial cells. It is the only member of the FGF family that binds with high affinity to all four FGF receptors (2). aFGF binds to cell surface receptors with high affinity with the prerequisite association with heparin sulfate. This ligation subsequently initiates receptor dimerization, transphosphorylation, as well as internalization of receptor/FGF complexes, and thus aFGF is translocated across cellular membranes and transported to the nucleus. The FGF pathway regulates primitive hematopoiesis by modulating transcription factors such as Gata1 expression level and activity (3). aFGF has been implicated in an autocrine system by which calcium regulates parathyroid cell growth. It has been demonstrated that the expression of aFGF is highest during the late stages of hepatic morphogenesis in newborn rats as well as during hepatic differentiation in adult liver. The intravenous application of aFGF has shown that the factor promotes the regeneration of the endothelium following arterial intravascular injuries (4). Overexpression of aFGF in pancreatic cancers has been found to be associated with a more advanced tumor stage. Recent studies have also demonstrated that chimeric toxins composed of aFGF fused to mutant forms of Pseudomonas exotoxin, are cytotoxic to a variety of tumor cell lines with FGF receptors (5). Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <0.1 EU/µg as determined by the LAL method Protein Accession No. Amino Acid Sequence aegeittft altekfnlpp gnykkpklly csngghflri lpdgtvdgtr drsdqhiqlq lsaesvgevy ikstetgqyl amdtdgllyg sqtpneeclf lerleenhyn tyiskkhaek nwfvglkkng sckrgprthy gqkailflpl pvssd
N-terminal Sequence Analysis Ala2 State of Matter Lyophilized Predicted Molecular Mass The predicted molecular weight of Recombinant Human β-ECGF is Mr 17 kDa. Predicted Molecular Mass 17 Formulation This recombinant protein was lyophilized from a 0.2 μm filtered solution in MOPS, sodium sulphate (Na2SO4), ethylenediaminetetraacetic acid (EDTA), and dithiothreitol (DTT). Storage and Stability This lyophilized protein is stable for six to twelve months when stored desiccated at -20°C to -70°C. After aseptic reconstitution, this protein may be stored at 2°C to 8°C for one month or at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day Ambient NCBI Gene Bank References & Citations1. Jaye, M. et al. (1986) Science 233:541 2. Otlewski, J. et al. (2009) Acta. Crystallogr. D. Biol. Crystallogr. 65:67 3. Nakazawa, F. et al. (2006) Blood. 108:3335 4. Bjornsson, TD. et al. (1991) Proc. Natl. Acad. Sci. (USA) 88:8651 5. Merwin, JR. et al. (1992) Cancer Res. 52:4995 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.