What are Immunoglobulins?
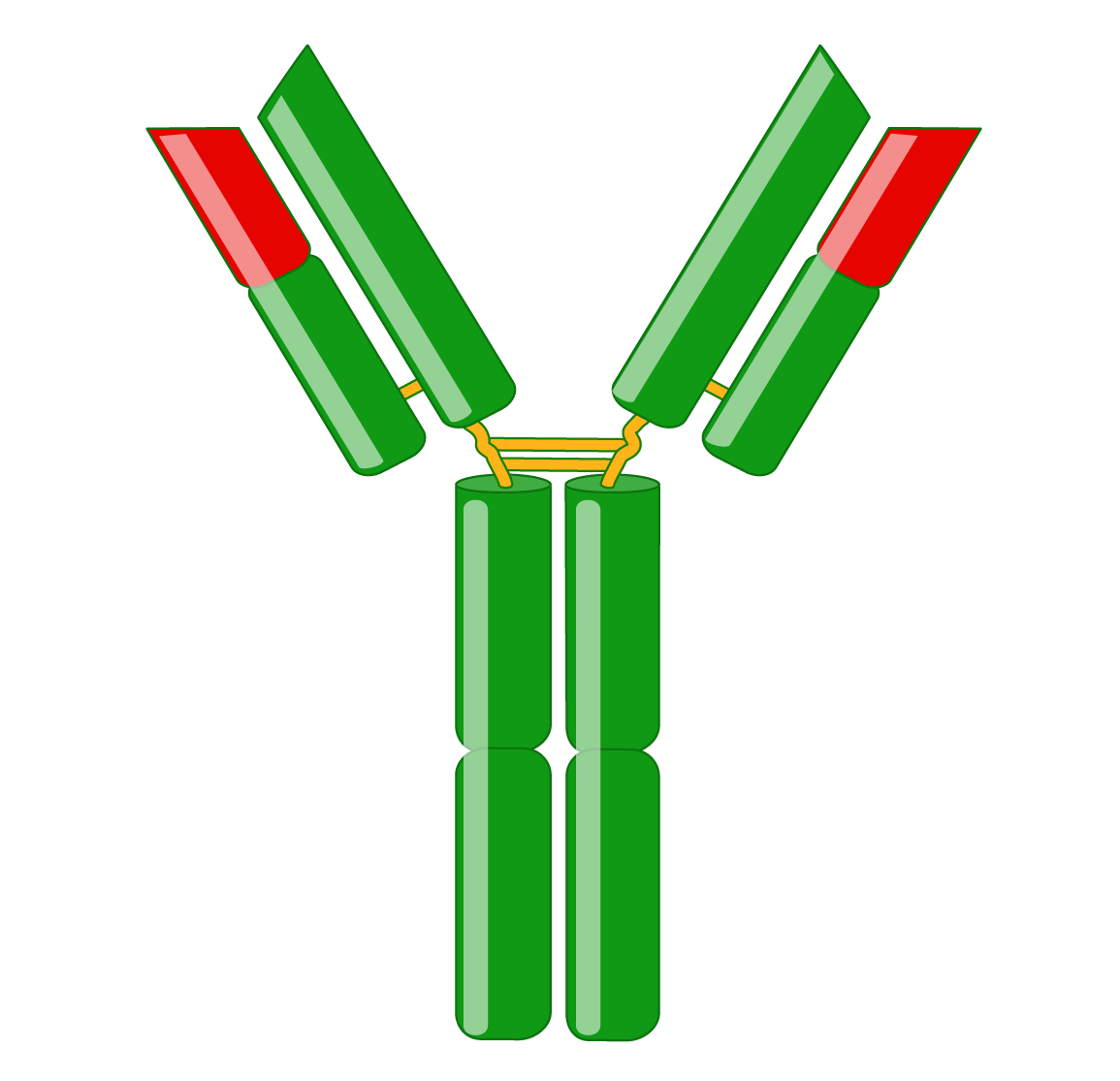
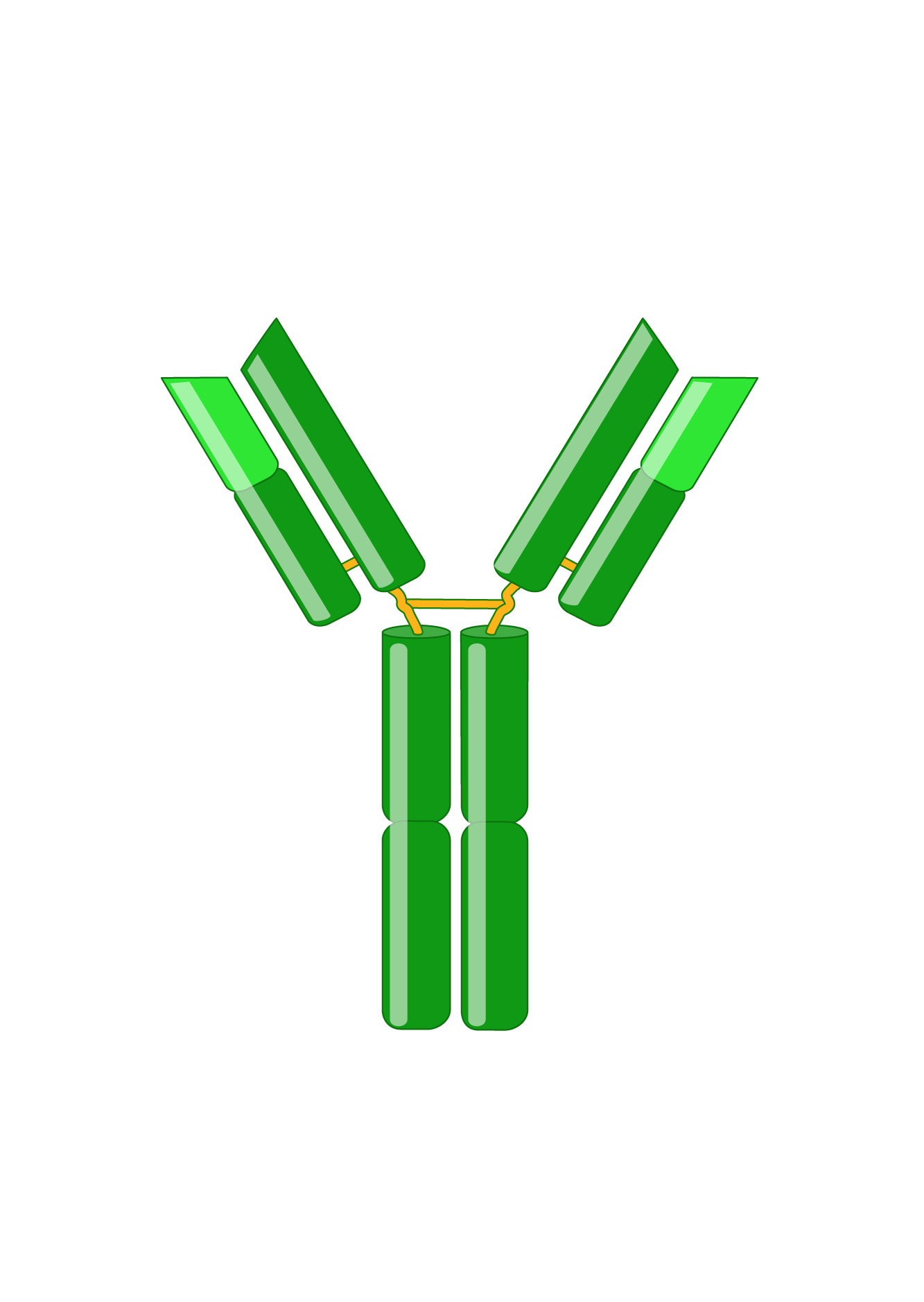
Antibodies, also known as immunoglobulins (Igs), are Y-shaped glycoproteins critical in the immune system’s defense against infection. Following B cell recognition of a foreign antigen by its B-cell receptor, B cells differentiate into plasma cells that produce and secrete antibodies, which have the same specificity as the receptor. Antibodies bind to antigens with high specificity and affinity, resulting in several effector functions, such as complement activation, enhanced phagocytosis (opsonization), and mast cell activation. Immunoglobulins are indispensable tools for research, diagnostics, and therapies.
Light Chain
Immunoglobulins consist of two identical heavy and two identical light chains. A light chain has an N-terminal variable region and C-terminal constant region. The variable region determines antigen-binding and is created by complex gene rearrangements, followed by somatic hypermutation after antigen exposure, resulting in three regions of sequence hypervariability (complementary-determining regions; CDRs) distributed between four constant sequences (framework regions; FRs). In mammals, there are two types of light chain, λ and κ.
Heavy Chain
Heavy chains also have a variable region for antigen-binding and three or four constant regions that mediate specific effector functions. Heavy chains with three constant regions also have a hinge region, which adds flexibility. There are five types of mammalian heavy chains, μ, γ, α, ε, and δ.
Classes of Immunoglobulins
Antibodies are classified into five isotypes based on these heavy chains as IgM, IgG, IgA, IgE, and IgD, respectively. Each isotype is unique in its structure, localization, and biological functions.
IgG is the most predominant isotype found in human serum, has the longest serum half-life, and controls infections in tissues. IgG has many functions, including activation of the classical complement pathway, opsonization, neutralization of toxins, and antibody-dependent cell-mediated cytotoxicity (ADCC). IgG is the only isotype that can cross the human placenta and enter the fetal circulation, protecting the fetus and newborn. There are 4 subclasses of IgG named in order of relative abundance (IgG1, IgG2, IgG3, and IgG4) that have different biological properties. IgG1 and IgG3 antibodies typically recognize protein antigens, while IgG2 recognizes polysaccharide antigens. IgG4 can bind to allergens and chronically persistent antigens and is considered anti-inflammatory. Due to its specificity, abundance, and longevity, IgG is the most commonly used isotype in research and diagnostics.
- Size: 150 kDa
- Light chain: λ or κ
- H-chain: γ1, γ2, γ3, γ4 – variable domain, 3 constant domains, and a hinge domain
- Number of antigen binding sites: 2
- Percent of total Ig: 75%
- Serum half-life: 7-23 days, depending on the subclass
- Structure: monomer
- Distribution: bloodstream and extracellular fluid
- Function: secondary response
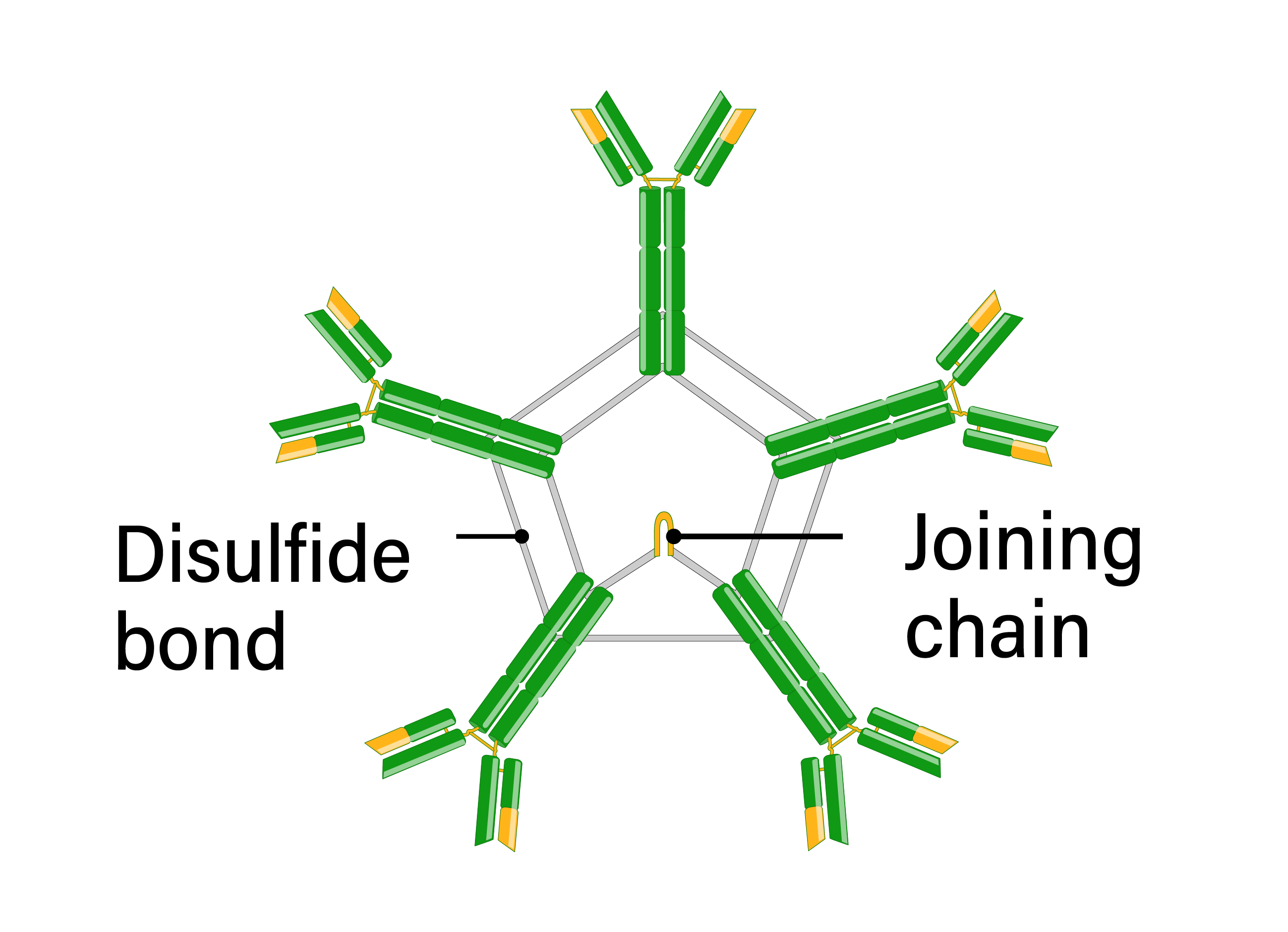
IgM immunoglobulin is the first isotype expressed during B cell development and following B cell activation. Naïve B cells express IgM monomers on their surface. Antigenic stimulation results in IgM secretion in a pentameric form, linked by disulfide bonds in the constant heavy regions. IgM is produced before class switching and somatic hypermutation and has a low affinity. However, the secreted pentameric form provides 10 antigen-binding sites and thus has high overall avidity. Due to the large size of the IgM pentamers, IgM is primarily found in the bloodstream, although it is present in the lymph to a lesser extent. IgM is especially effective at activating the classical complement system, leading to opsonization of antigens and cytolysis. Clinically, IgM is frequently used in diagnostic assays for acute infections since it is involved in the primary immune response.
- Size: 900 kDa
- Light chain: λ or κ
- Heavy chain: µ – variable domain and 4 constant domains
- Number of antigen binding sites: 10
- Percent of total Ig: 10%
- Serum half-life: 5 days
- Structure: pentamer
- Distribution: mainly in bloodstream
- Function: primary response
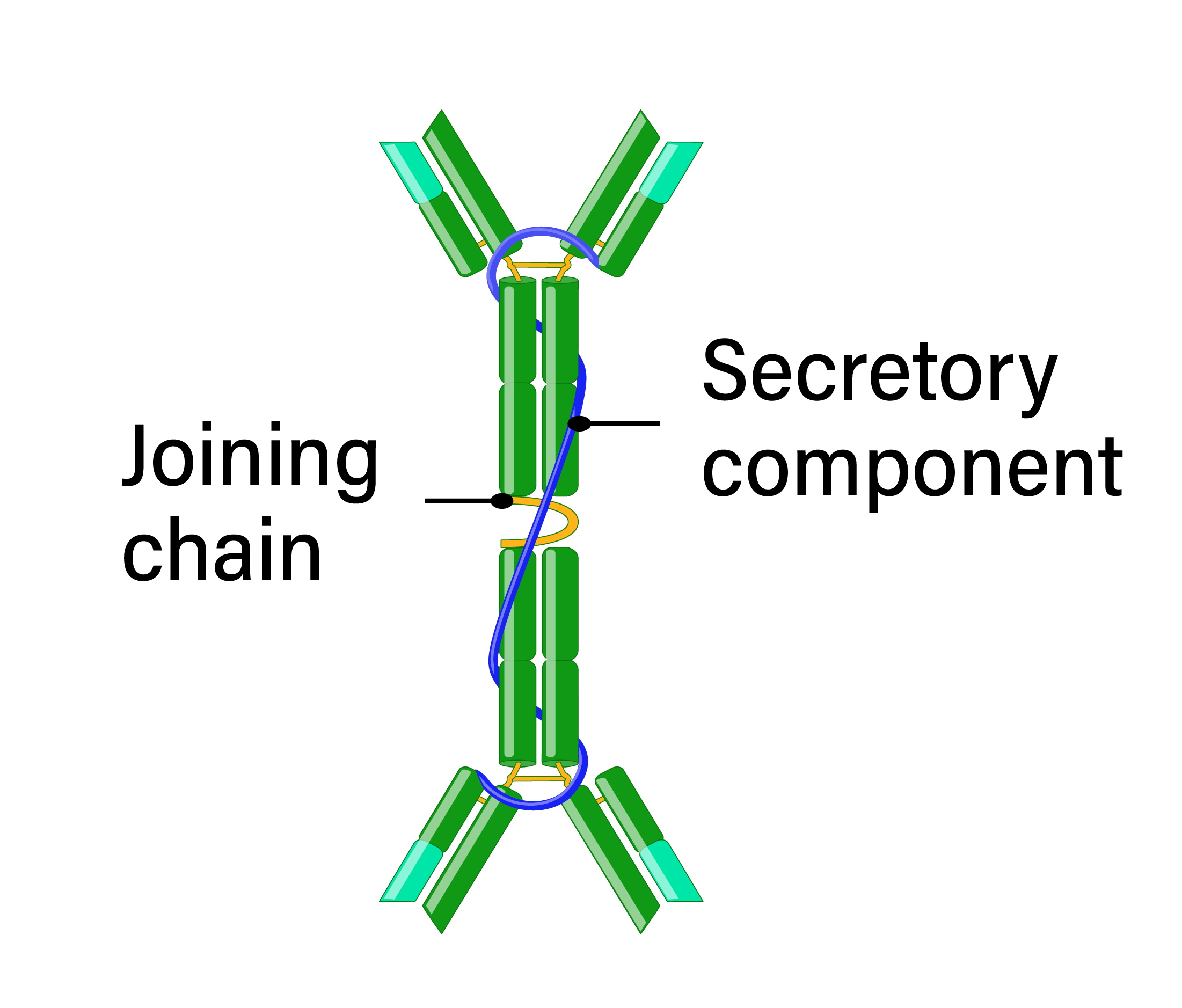
IgA is present in serum, mucosal surfaces, and secretions, including saliva and breast milk. IgA antibodies are either monomeric in serum or secreted as dimers in the mucosa, connected by a joining peptide. There are two subclasses of IgA, IgA1 and IgA2, which differ at their hinge regions resulting in differing sensitivities to bacterial proteases. Because of its localization at mucosal surfaces, IgA often provides the primary defense mechanism against infections by direct neutralization or by preventing attachment and penetration of epithelial surfaces. IgA is not a potent opsonin and does not activate complement; however, neutrophils express the IgA receptor, suggesting a role in ADCC.
- Size: 160 kDa (monomer)
- Light chain: λ or κ
- Heavy chain: α1, α2 – variable domain, 3 constant domains, and a hinge domain
- Number of antigen binding sites: 4
- Percent of total Ig: 15%
- Serum half-life: 6 days
- Structure: monomer or dimer
- Distribution: bloodstream and secretions
- Function: immunity at mucus membranes
IgD antibodies mostly exist as monomers coexpressed with IgM on the surface of mature B cells, suggesting IgD regulates B cell fate at specific developmental stages. IgD is also present at low levels in the serum. However, IgD antibodies do not participate in the major antibody effector mechanisms, and the function of circulating IgD is mostly unknown.
- Size: 180 kDa
- Light chain: λ or κ
- Heavy chain: δ – variable domain, 3 constant domains, and a hinge domain
- Number of antigen binding sites: 2
- Percent of total Ig: 0.2%
- Serum half-life: 3 days
- Structure: monomer
- Distribution: B cell surface
- Function: unknown
IgE antibodies are the least abundant isotype and have the lowest serum half-life. However, they avidly bind to receptors on mast cells and basophils beneath the skin and mucosa and along blood vessels in connective tissue. Antigen binding results in mast cell release of chemical mediators, such as coughing and sneezing, that expel infectious agents, including multicellular parasites. IgE antibodies are also involved in common allergic diseases and used in diagnostic assays for allergy.
- Size: 200 kDa
- Light chain: λ or κ
- Heavy chain: ε – variable domain and 4 constant domains
- Number of antigen binding sites: 2
- Percent of total Ig: 0.002%
- Serum half-life: 2 days
- Structure: monomer
- Distribution: bound to mast cells and basophils
- Function: immunity against parasites
F(ab) and Fc fragments
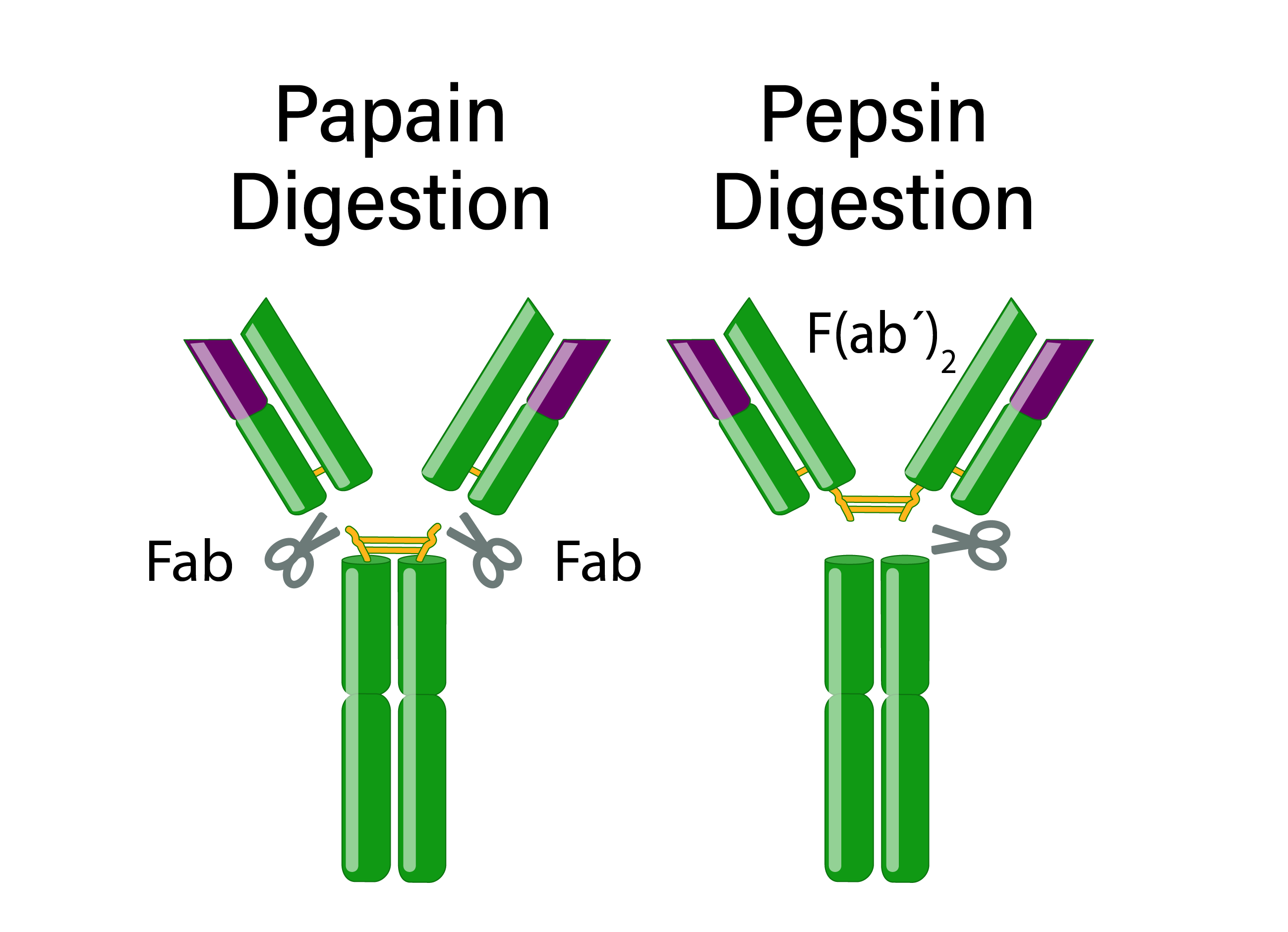
Immunoglobulins consist of two F(ab) fragments and one Fc fragment with distinct characteristics. Proteases can fragment antibodies to allow for specific experimental applications.
F(ab) fragments
An antibody has two identical F(ab) fragments (50 kDa), which contain the entire light chain and the variable and first constant domain of the heavy chain and are responsible for antigen-binding. Antibody digestion with papain generates two monovalent F(ab) fragments with only one antigen-binding site. In contrast, pepsin digestion occurs below the hinge region, resulting in a divalent F(ab’)2 fragment with two antigen-binding sites that can cross-link antigens.
Fc fragments
The Fc fragment (50 kDa) is composed of the heavy chain constant region of an antibody. The Fc fragment binds to endogenous Fc receptors on the surfaces of immune cells and thus mediates an antibody’s effector functions.
References:
Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41-S52. doi:10.1016/j.jaci.2009.09.046
Murphy, K., Weaver, C., & Janeway, C. (2017). Janeway’s immunobiology