In vivo grade antibodies have a variety of uses in biological research, from discovery of cell signaling pathways to manipulation of biological systems in animal models for pre-clinical studies. In vivo antibodies from Leinco Technologies have the highest purity standards in the industry, with low endotoxin levels, no detectable aggregates nor leachable protein A. These antibodies are produced in our cGMP and ISO 9001:2015 / ISO 13485:2015 certified facility and are available in custom concentrations and package sizes. In vivo PLATINUM antibodies are also suitable for animal injection as they are pathogen free as determined by the IDEXX Impact I PCR mouse pathogen profile.
Leinco Technologies offers a broad range of purified in vivo antibodies that can be utilized in flow cytometry, immunohistochemistry, spatial biology studies, and more. Compare and contrast in vivo GOLD™ vs. in vivo PLATINUM™ to see how they measure up to our quality standards! Antibodies are available in 1 mg, 5 mg, 25 mg, 50 mg and 100 mg sizes. Bulk sizes, custom concentrations and custom packaging are available on request.
Categories
Filters
GOLD™ vs. PLATINUM™ in vivo Antibody Quality Standards
| Specifications |  | |
|---|---|---|
| Binding validation determined by WB, FC or ELISA | Yes | Yes |
| Endotoxin level determined by the LAL method | ≤ 1.0 EU/mg | ≤ 0.5 EU/mg |
| Antibody aggregation screening by analytical SEC | ≥ 95% monomer | ≥ 98% monomer |
| Purity by SDS PAGE | ≥ 95% | ≥ 98% |
| Formulation for in vivo use | No preservatives No stabilizers No carrier protein Sterile PBS pH 7.2 – No K+ or Ca2+ Concentration: > 5mg/mL | No preservatives No stabilizers No carrier protein Sterile PBS pH 7.2 – No K+ or Ca2+ Concentration: > 5mg/mL |
| Murine pathogen screening | Not applicable, see PLATINUM functional grade antibodies | Pathogen tested (IMPACT1) (see table) |
| Applications | In vivo functional studies and may be used for WB, FC, IF or IHC | |
| Product preparation | Functional grade preclinical antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. | |
| Storage and handling | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. • 1 month, 2 to 8 °C under sterile conditions, as supplied. • 12 months, -70 °C under sterile conditions. | |
IDEXX IMPACT I (PCR-based) Mouse Pathogen Profile for Purified In vivo PLATINUM™ Grade Antibodies
| Mycoplasma sp. | Mouse adenovirus 1 (MAV1) | Lactate dehydrogenase-elevating virus (LDEV) |
| Mycoplasma pulmonis | Mouse adenovirus 2 (MAV2) | Mouse kidney parvovirus (MKPV) |
| Sendai virus | Murine norovirus (MNV) | Hantaan Virus |
| Mouse hepatitis virus (MHV) | Reovirus 3 (REO3) | Mouse cytomegalovirus (mCMV) |
| Pneumonia virus of mice (PVM) | Mouse rotavirus (EDIM) | K virus |
| Minute virus of mice (MVM) | Ectromelia virus | Mouse thymic virus (MTV) |
| Mouse parvovirus (MPV) | Polyomavirus | Corynebacterium bovis |
| Theiler’s murine encephalomyelitis (TMEV) | Lymphocytic choriomeningitis virus (LCMV) | Corynebacterium sp. |
Bioanalytical Data
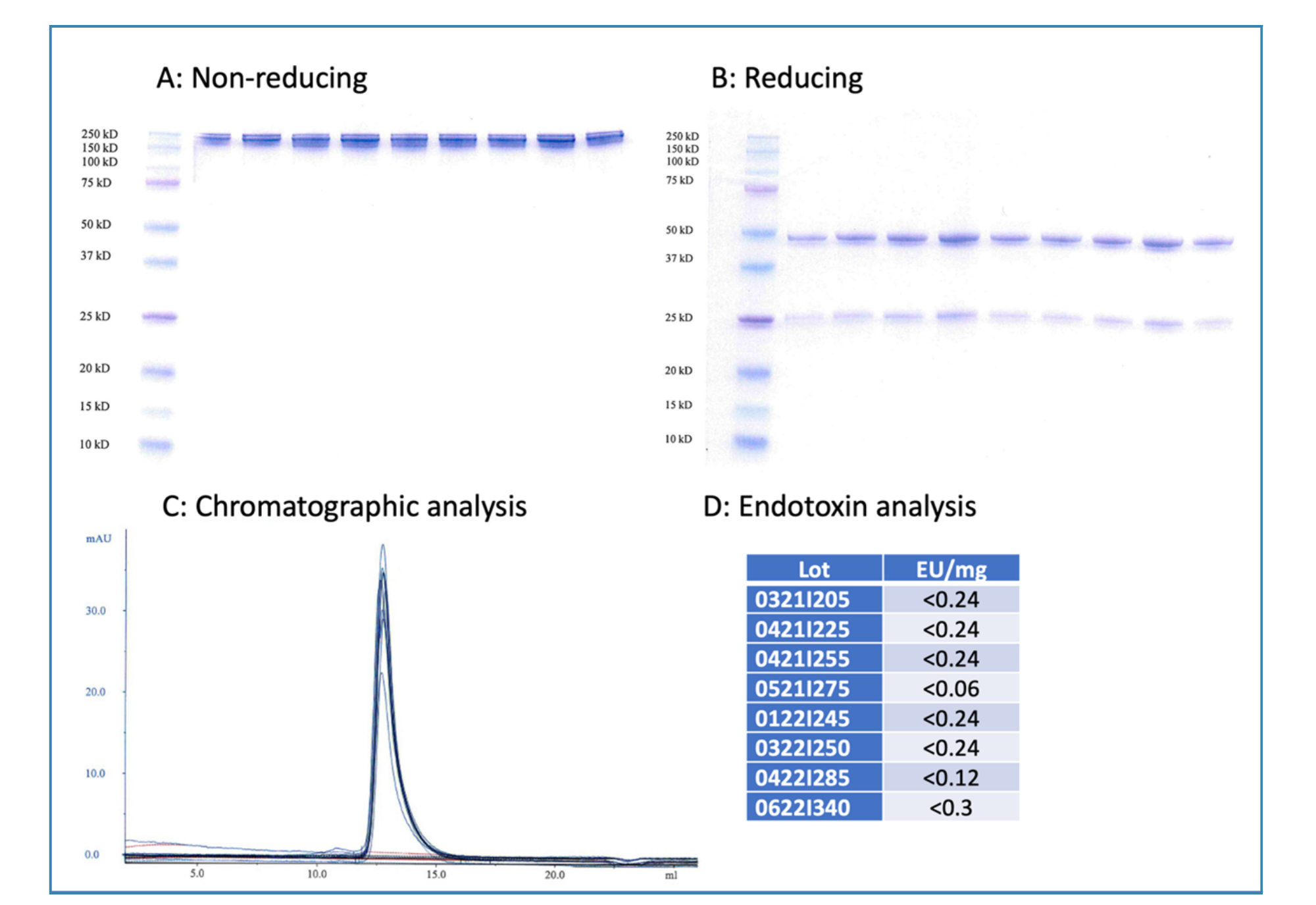
Figure 1. Quality control of in vivo antibodies: RMP1-14
In vivo antibodies from Leinco Technologies are subjected to extensive QC to ensure high reproducibility between lots. The example in Figure 1 shows the results for different lots of RMP1-14 antibody, directed against PD-1. The lots have consistently high purity as indicated by non-reducing and reducing SDS-PAGE (Fig. 1A and 1B, resp.). Analysis by size exclusion chromatography gave superimposing chromatograms for all nine lots, with overlapping retention times and minimal baseline noise (Fig. 1C). Endotoxin data for eight lots indicated that all lots were below the specification of ≤ 0.5 EU/mg as determined by the limulus amebocyte lysate (LAL) method (Fig. 1D).

