Lateral Flow Tests
Lateral flow assay development is the successful production of a simple to use diagnostic test for validating the presence or absence of a wide range of pathogens, biomarkers and antibodies in a specific species. Rapid lateral flow tests are used for qualitative and quantitative analysis. Qualitative by visually indicating if a substance is present and quantitative by determining how much of that substance is present. Lateral flow immunoassay tests are highly important and versatile within point-of-care diagnostics.
How Do Lateral Flows Work?
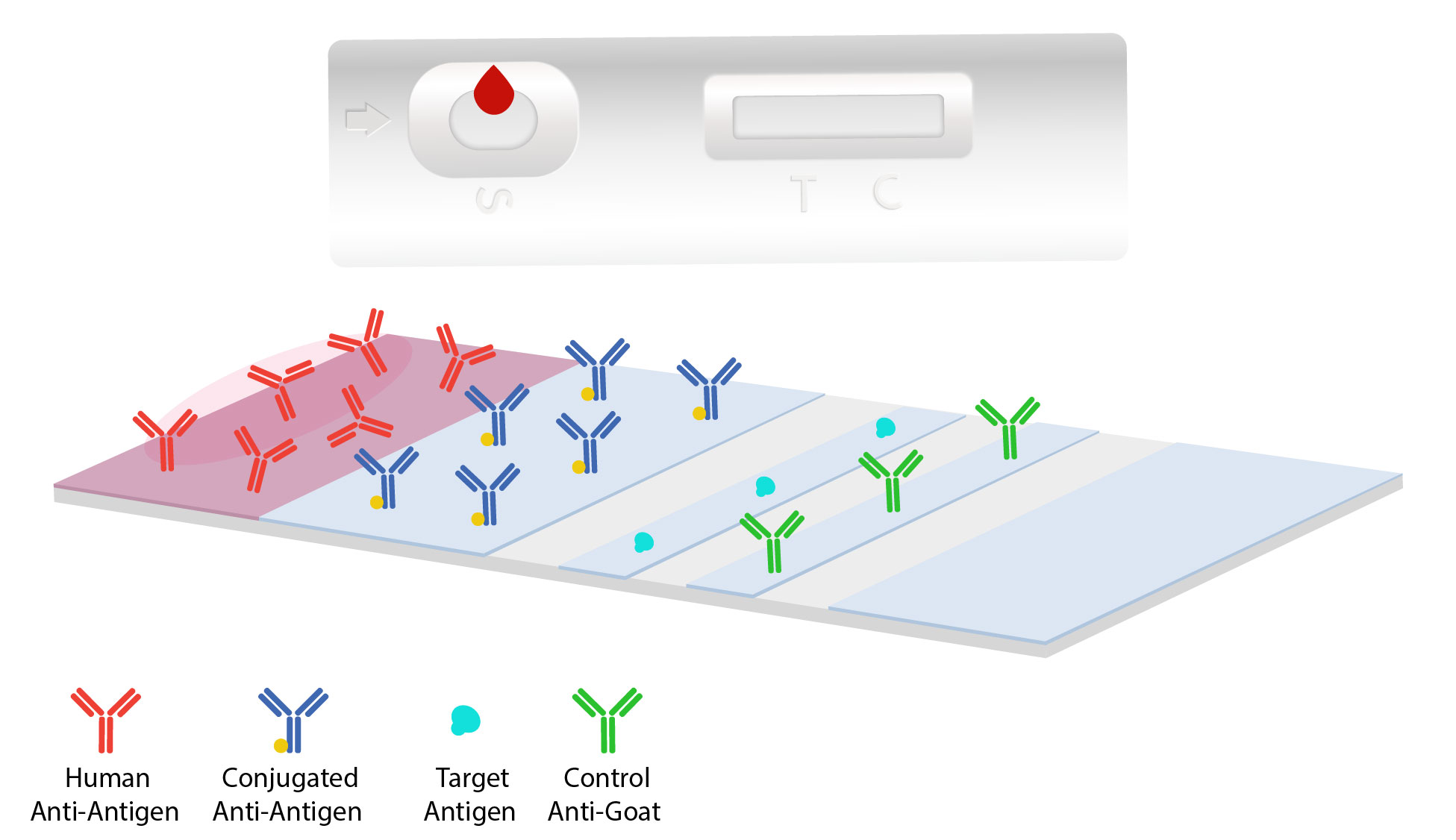
Components of the test flow laterally through the devices grid allowing for antigens to combine with antibodies to signal a response between the complexes. The components involved in a lateral flow assay are the sample pad, a conjugate pad, a nitrocellulose membrane, and a absorbent pad.
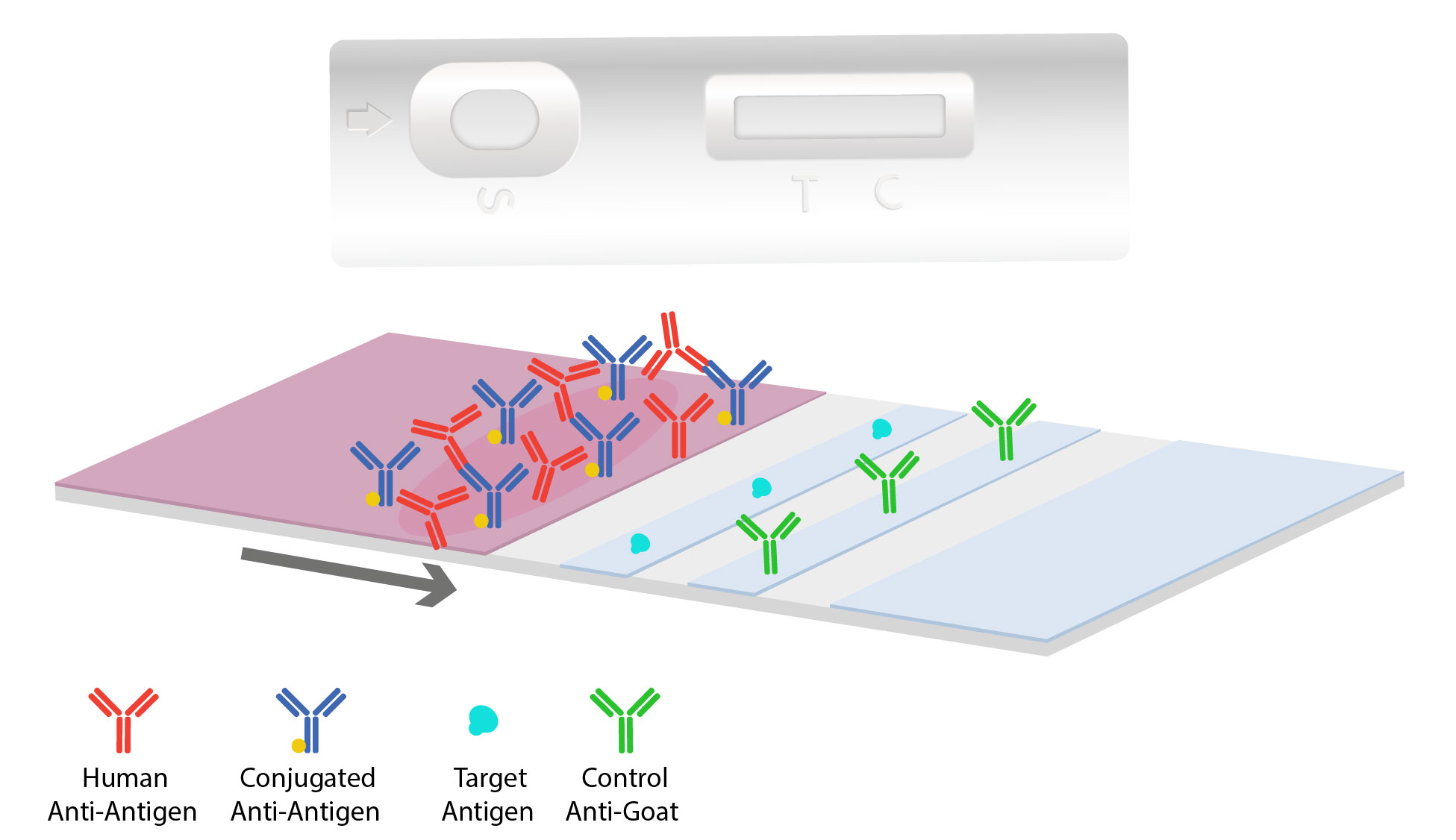
The sample moves onto a conjugate pad. This pad is typically coated with the desired conjugated antibody, such as colloidal gold or other fluorescent particles. If the target is present, the immobilized conjugated antibodies and labels will bind to the target forming complexes and continue to migrate along the test.
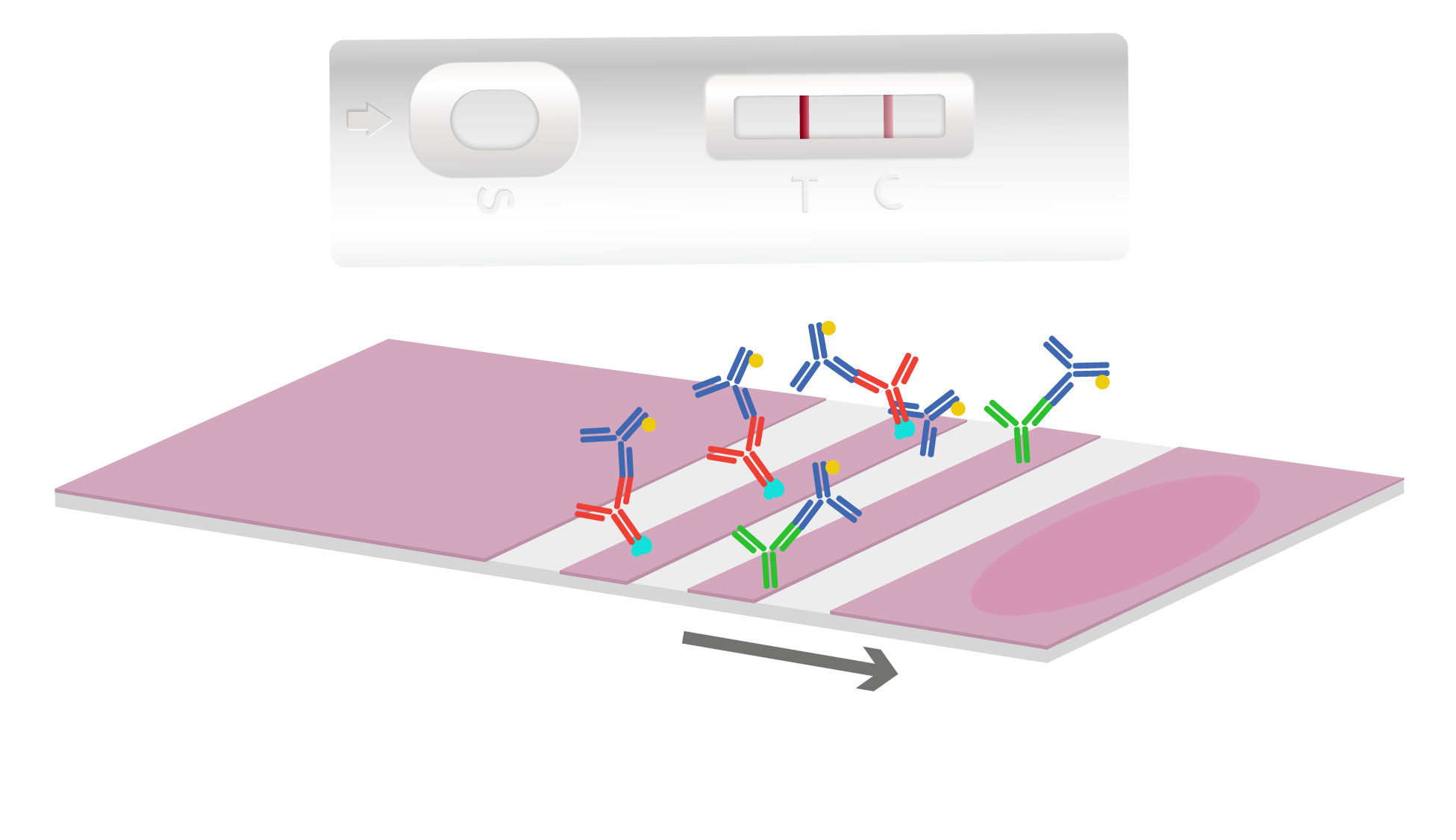
As the sample and antibody complex moves onto the antigen-coated nitrocellulose test line, interaction occurs. If the target antigen is present, a colored line will form and the density of the line depends on the quantity of that antigen present. To quantify and determine target concentration results, a rapid test can be combined with a digital reader. When there are no antibodies specific to the antigen, this results in no colored line to develop indicating a “negative” test. 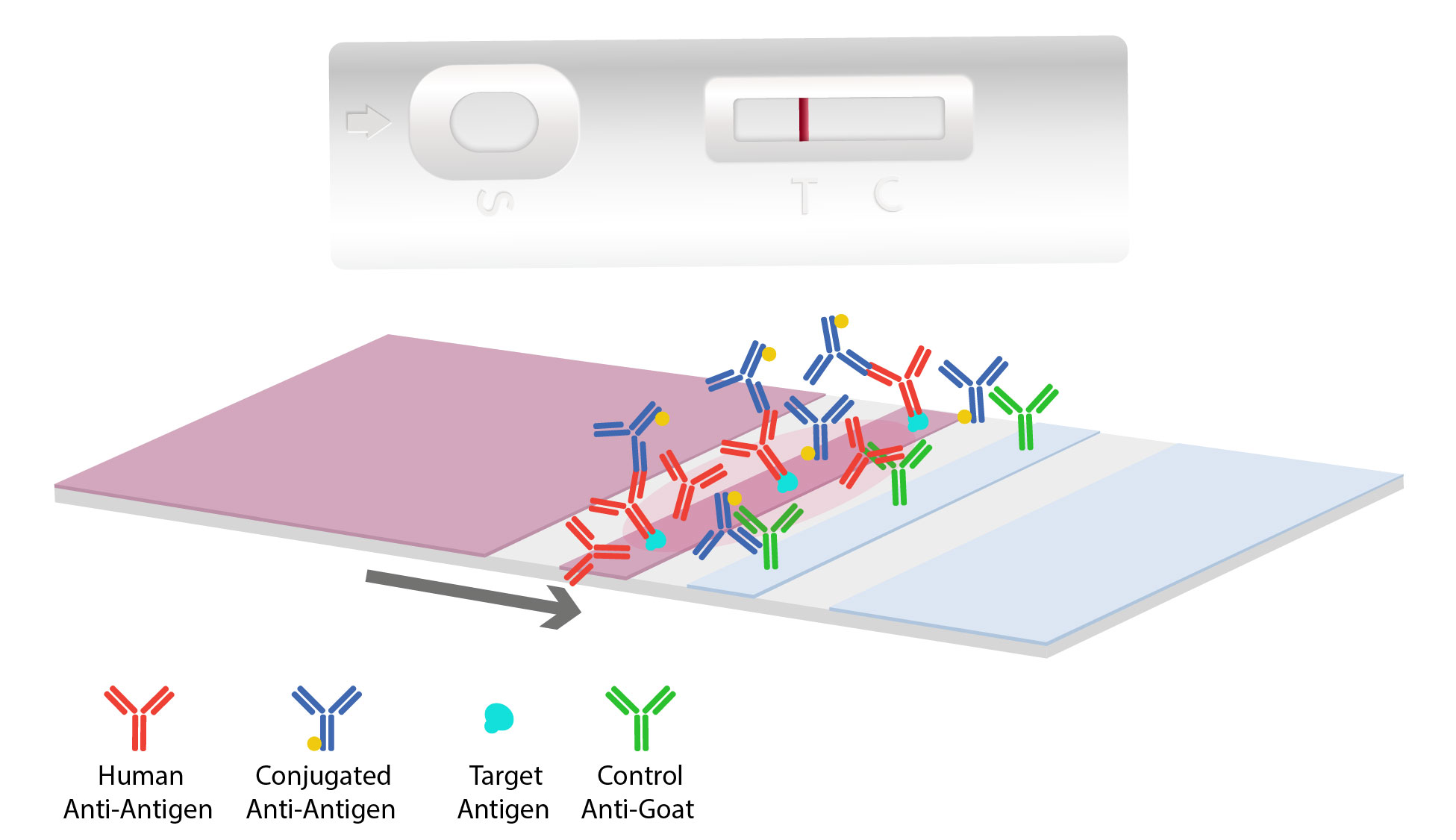
Most tests will contain a second line, called the control line, which is coated with antibodies to pick up the excess gold conjugates particles. This results in a second colored line indicating the flow test operated correctly. 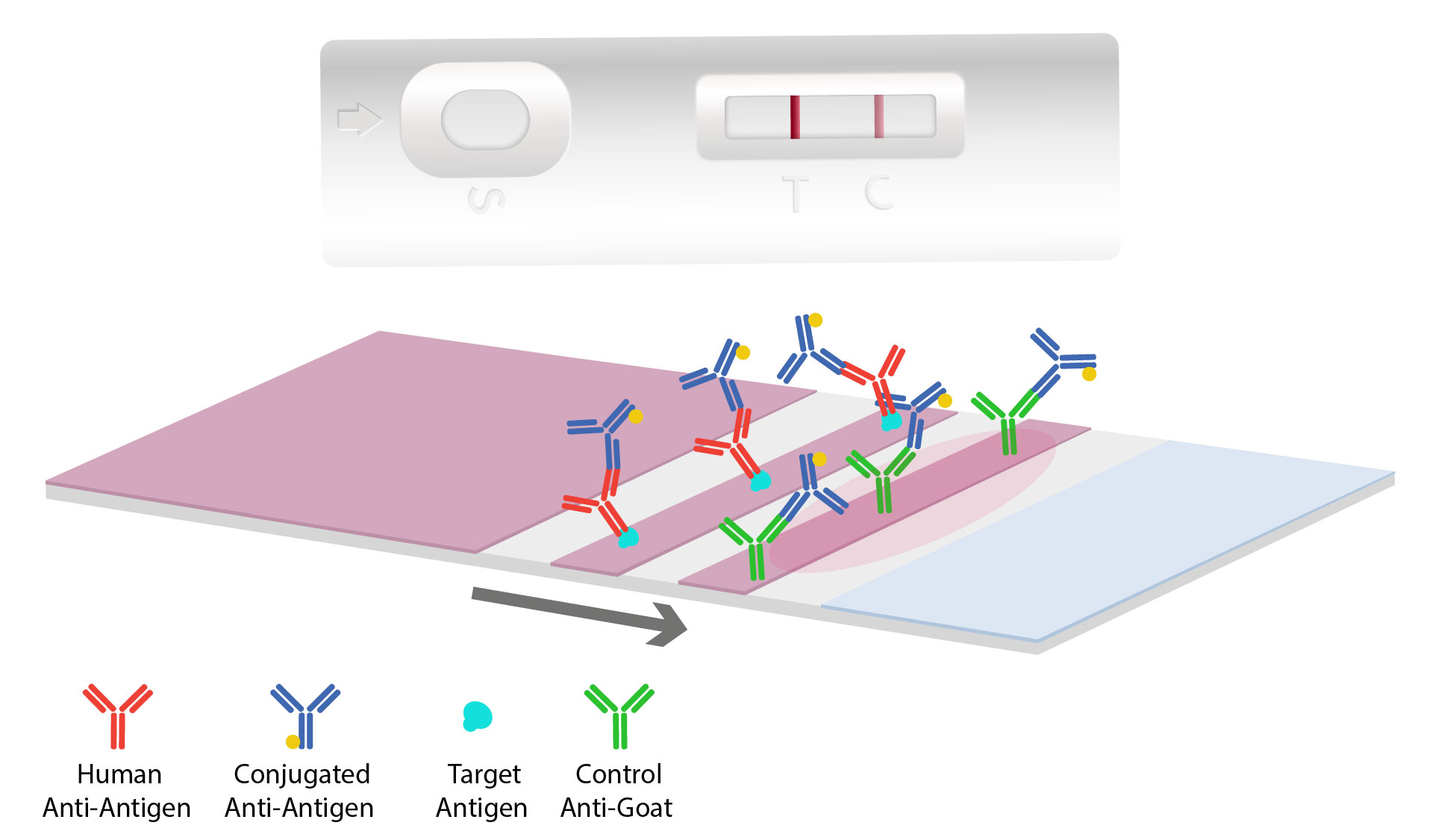
The sample will then reach an absorbent pad. This primary function of the absorbent pad is to allow an increase in the total sample volume to enter the test strip. This increased volume can be used to wash unbound detector complexes away from the test and control line, therefore lowering potential background and enhancing test sensitivity.
Immunoassay Development Services with Leinco
Our experienced research and scientific team assists in rapid point-of-care lateral flow assay development from the planning to validation phase. Leinco provides high quality assay development services and products while meeting rigorous deliverables and specifications. Antibodies used in lateral flow tests should have high sensitivity, specificity, purity, and stability to accomplish performance requirements. We work closely with customers throughout every step of their assay development process, assuring our components are optimized for your IVD diagnostic and research needs.