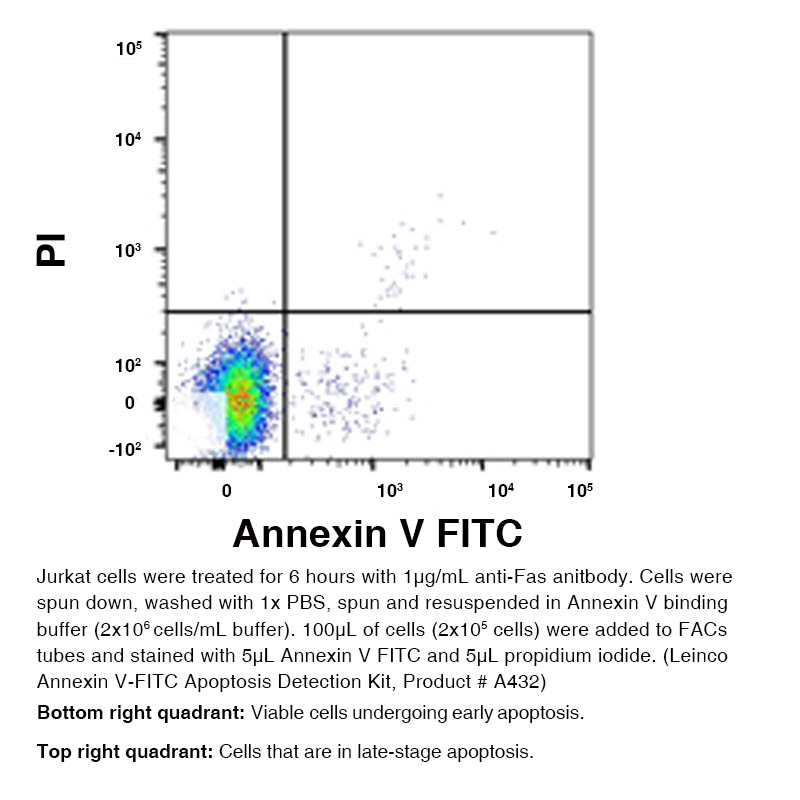
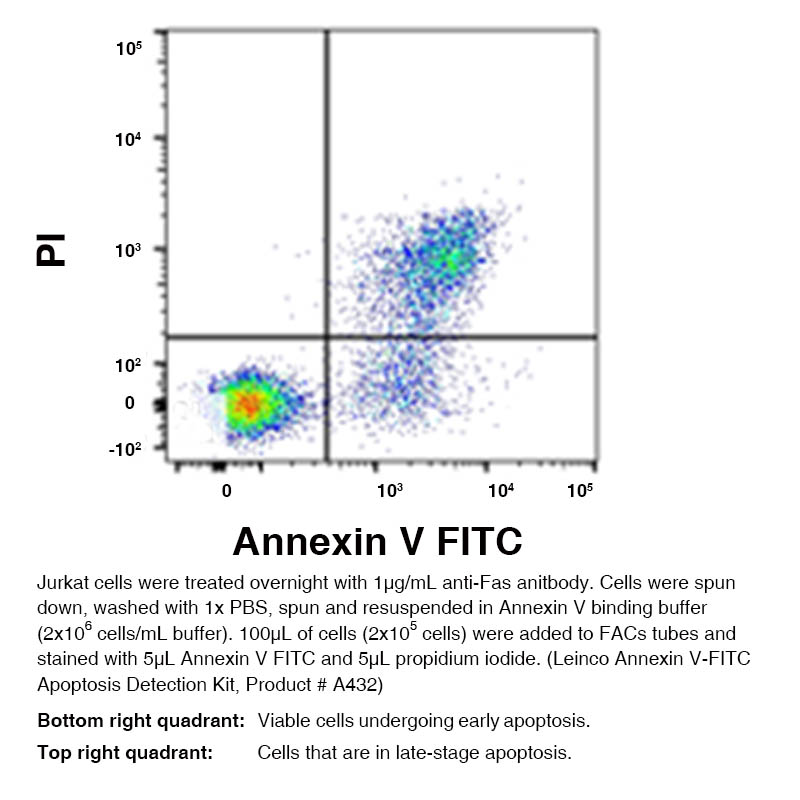
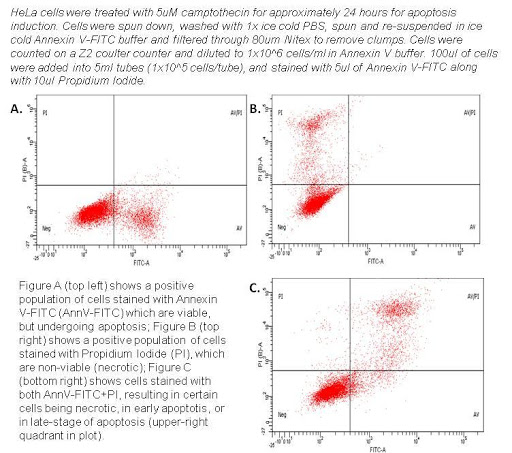
Annexin V – FITC Apoptosis Detection Kit
Data
- -
- -
DescriptionBackground Apoptosis, the physiological process of cell death, is a naturally occurring phenomenon. It is normally a tightly regulated process; however, if deregulated, tumor growth can result. Apoptotic processes can be measured in the laboratory easily and most effectively by using a FITC-labeled Annexin V protein to stain cells and analyze them via flow cytometry. It is a useful method to screen and analyze cells in a high throughput manner, resulting in a clearer understanding of the cellular response to various stimuli. When cells are undergoing cell death, phosphatidylserine (PS), a phospholipid which makes up a portion of the cell membrane, will translocate to the outside-facing side of the membrane. This translocation in the cell death mechanism exposes PS to the extracellular environment. Annexin V, a 35-36 kDa phospholipid-binding protein, has a high affinity for PS (KDa=7nmol/l). Once Annexin V is bound to the apoptotic cell it acts as a beacon for cells that have undergone apoptosis. Annexin V is often conjugated to FITC so the amount of signal can be measured and analyzed from labeled cells using a flow cytometer. The translocation of PS to the outside or exposed side of the membrane is an early event in the apoptotic process. Therefore, Annexin V staining is considered a marker for early stage apoptosis. Often times, Annexin V is paired with Propidium Iodide (PI) stain in order to analyze for late-apoptotic or dead cells. Early stage apoptotic cells will only take up the Annexin V stain but will remain PI negative, and late-stage apoptotic cells will be positive for both Annexin V and PI. Directions Three control samples should be used to calibrate the instrument. First, unstained cells resuspended in binding buffer only should be assessed to evaluate the level of autofluorescence and to adjust the instrument accordingly. Then, treated cells should be single stained with Annexin V-FITC and Propidium Iodide to define the boundaries of each population. Apoptotic cells labeled with Annexin V- FITC should appear in the lower half of the dot plot, with no events accumulating in the upper left or upper right quadrants. Similarly, cells labeled with propidium iodide alone should show no events in the upper or lower right quadrants. Annexin V-FITC is provided at 100X final concentration. The following steps should be followed as a general guide. A higher or lower final concentration of Annexin V conjugate may be required. Approximately 1x105 to 1x106 cells should be processed per 100 μl of labeling reagent (see below). Adherent cells may be released from their substrate using 0.25% trypsin or 0.02 to 2% EDTA in PBS or HBSS. Care should be taken when trypsinizing to prevent excessive cell damage. It can help to keep trypsinized cells in the presence of 2% BSA to prevent further damage when processing these samples. When using EDTA, it is necessary to remove all EDTA by washing twice in 1X PBS or 1X binding buffer prior to labeling to avoid chelating the calcium necessary for annexin binding. Kit DetailsMaterials Provided 1.) Annexin V-FITC - Formulated in 0.01 M PBS pH 7.4, 150 mM NaCl, 1% BSA and 0.09% sodium azide as a preservative. 2.) Propidium Iodide (PI) - Formulated in 0.01 M PBS pH 7.4, 150 mM NaCl. Caution: Propidium Iodide Solution is toxigenic and mutagenic; Please handle with care. 3.) Binding Buffer (no preservative added) Storage Stable when stored dark at 2°-8°C. Do Not Freeze. Other Materials and Solutions Required Equipment Required 1.) Fluorescence Microscope or Flow Cytometer (Capable of FITC and Propidium Iodide Detection) 2.) Microcentrifuge 3.) Adjustable Pipettors (100-1000 μl, 10-200 μl, and 1-20 μl) 4.) Ice Bucket and Ice Other Reagents and Disposables Needed 1.) PBS Buffer (Leinco Part No.: P349 or P364) 2.) Microcentrifuge Tubes 3.) Aluminum Foil 4.) Gloves Assay Procedure Suggested Staining Protocol for Jurkat Cells 1.) Collect cells by centrifugation at a 100 - 300 g for 5 – 15 minutes at room temperature all depending on cell type. NOTE: For Jurkat Cells we grow cells to log phase with a viability of > 95% and upon log phase, we count cells and centrifuge at 1000 rpm for 5 minutes at room temperature. 2.) Resuspend in fresh media at 1×106 and add 1 µg per ml of anti-human CD95 (anti-Fas) antibody and culture cells overnight in 37°C incubator with 5% CO2 for best results. 3.) After incubation, centrifuge cells at 1000 rpm for 5 minutes at room temperature and remove supernatant when finished. Wash cells for analysis using cold 1X PBS twice. 4.) Gently resuspend at 2×106 cells / ml in 1X Binding Buffer. 5.) Transfer 100 μL of cell suspension to desired tubes suited for flow cytometry analysis. 6.) Add 5 µL of Annexin V-FITC and 2.5 - 5.0 µL (1/4 of what is shown below) of PI to the cell suspension and gently mix. 7.) Incubate for 10-15 minutes in the dark at room temperature. (Optional: samples may be washed and re-suspended in chilled PBS, if desired depending on cell type) 8.) Perform analysis using flow cytometer within 45 minutes following incubation. DO NOT USE FIXATIVE Troubleshooting Technical Tips and Tricks
1.) Annexin V binding is calcium-dependent, and defined calcium and salt
concentrations are required for optimal staining. Always use the supplied
Annexin V binding buffer for staining.
2.) We recommend using a positive control such as camptothecin to induce
apoptosis in cells being tested. Technical ProtocolsReferences & Citations |