Anti-Bovine PKC Protein Kinase C Purified
Anti-Bovine PKC Protein Kinase C Purified
Product No.: P107
- -
- -
Clone MC5 Target Protein Kinase C Formats AvailableView All Product Type Monoclonal Antibody Isotype IgG Applications IHC FF , IHC FFPE , IP , N , WB |
Data
- -
- -
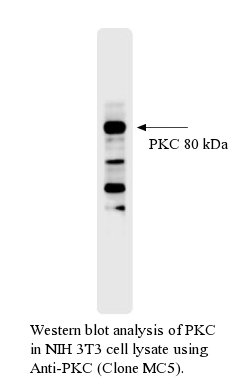
Antibody DetailsProduct DetailsReactive Species Bovine Host Species Mouse Immunogen Purified Recombinant Bovine PKC (>98%) Product Concentration 0.5 mg/ml Formulation This purified antibody is formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.4, 1% BSA and 0.09% sodium azide as a preservative. Storage and Handling This purified antibody is stable when stored at 2-8°C. Do not freeze. Shipping Next Day Ambient RRIDAB_2831507 Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Anti-Protein Kinase C (PKC) recognizes the intact (Mr 79-80 kDa) calcium-activated, phospholipid-dependent Protein Kinase C in bovine brain. The antibody strongly cross-reacts with PKC in human, mouse and rat cells.1 Anti-PKC (clone MC5) binds to an epitope at the hinge region of the enzyme, the site of phorbol-ester-induced proteolysis which is thought to down-regulate PKC activity in intact cells.1,2 Monoclonal MC5 reacts with PKC-α/ß1/ß2 isoforms. Background Protein Kinase C (PKC) is a family of protein kinases consisting of ~10 isozymes. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, diacylglycerol (DAG), and a phospholipid such as phosphatidylcholine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for activation. Thus, conventional and novel PKCs are activated through the same signal transduction pathway as phospholipase C. On the other hand, atypical (a)PKCs (including protein kinase Mζ and ι / λ isoforms) require neither Ca2+ nor diacylglycerol for activation. The term "protein kinase C" usually refers to the entire family of isoforms. The structure of all PKCs consists of a regulatory domain and a catalytic domain tethered together by a hinge region. The catalytic region is highly conserved among the different isoforms, as well as, to a lesser degree, among the catalytic region of other serine/threonine kinases. The second messenger requirement differences in the isoforms are a result of the regulatory region, which are similar within the classes, but differ among them. Most of the crystal structure of the catalytic region of PKC has not been determined, except for PKC theta and iota. Due to its similarity to other kinases whose crystal structure have been determined, the structure can be strongly predicted. The regulatory domain or the amino-teminus of the PKCs contains several shared subregions. The C1 domain, present in all of the isoforms of PKC has a binding site for DAG as well as non-hydrolysable, non-physiological analogues called phorbol esters. This domain is functional and capable of binding DAG in both conventional and novel isoforms, however, the C1 domain in atypical PKCs is incapable of binding to DAG or phorbol esters. The C2 domain acts as a Ca2+ sensor and is present in both conventional and novel isoforms, but functional as a Ca2+ sensor only in the conventional. The pseudosubstrate region, which is present in all three classes of PKC, is a small sequence of amino acids that mimic a substrate and bind the substrate-binding cavity in the catalytic domain keeping the enzyme inactive. When Ca2+ and DAG are present in sufficient concentrations, they bind to the C2 and C1 domain, respectively, and recruit PKC to the membrane. This interaction with the membrane results in release of the pseudosubstrate from the catalytic site and activation of the enzyme. In order for these allosteric interactions to occur, however, PKC must first be properly folded and in the correct conformation permissive for catalytic action. This is contingent upon phosphorylation of the catalytic region, discussed below. PubMed Research Area Apoptosis . Cell Biology . Immunology References & Citations1. Young, S. et al. (1988) Eur. J. Biochem. 172:247 2. Young, S. et al. (1987) Biochem. J. 244:775 Technical ProtocolsCertificate of Analysis |
Formats Available
- -
- -
Prod No. | Description |
|---|---|
P321 | |
P322 | |
P323 | |
P325 | |
P326 | |
P327 | |
P107 | |
P108 | |
P109 | |
P110 |