Anti-Heme Oxygenase-1 Antibody (13061)
Anti-Heme Oxygenase-1 Antibody (13061)
Product No.: 13061
- -
- -
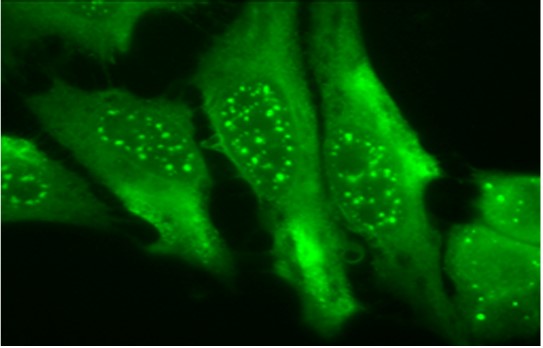
Clone 1F12.A6 Target Heme Oxygenase-1 Formats AvailableView All Product Type Monoclonal Alternate Names HO-1, EC 1.14.14.18 Isotype Mouse IgG1 Applications ELISA , WB |
Data
- -
- -
Antibody DetailsProduct DetailsReactive Species Bovine ⋅ Human ⋅ Mouse Host Species Mouse Immunogen Synthetic peptide corresponding to aa 1-30 of human HO-1. Product Concentration 1.0 mg/ml Formulation PBS, pH 7.4. State of Matter Liquid Product Preparation Purified by Protein G affinity chromatography Storage and Handling This antibody is stable for at least one (1) year at -20°C. Avoid repeated freeze-thaw cycles. Regulatory Status Research Use Only Country of Origin USA Shipping Next Day 2-8°C Applications and Recommended Usage? Quality Tested by Leinco ELISA: use at 1ug/ml with HO-1 on the solid phase.
Immunoblotting: use at 0.5-1ug/ml. A band of ~32kDa is detected. Immunofluorescence: use at 5-10ug/ml Positive control: Human cell line lysates such as A431, A549, HeLa, HepG2, Jurkat, or MCF7. These are recommended concentrations. Enduser should determine optimal concentrations for their applications. Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This antibody recognizes human, mouse, and bovine HO-1. It does not cross-react with HO-2. Background Heme-oxygenase is an enzyme that catalyzes heme catabolism to yield biliverdin, iron, and carbon monoxide (CO). There are three isoforms of heme-oxygenase: HO-1, HO-2, and HO-3. HO-1 and HO-2 have been identified as the two major isoforms in mammals. HO-1, also known as heat shock protein 32 (Hsp32) is induced by most oxidative stress inducers, cytokines, inflammatory agents, and heat shock. HO-1 deficiency appears to cause reduced stress defense, a pro-inflammatory tendency, susceptibility to atherosclerotic lesion formation, endothelial cell injury, and growth retardation. Therefore, up-regulation of HO-1 is one of the major defense mechanisms against oxidative stress. Function Heme oxygenase cleaves the heme ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of heme oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed. Exhibits cytoprotective effects since excess of free heme sensitizes cells to undergo apoptosis. NCBI Gene Bank ID UniProt.org Research Area Enzymes References & CitationsTechnical ProtocolsCertificate of Analysis |