Anti-Human EGFR (Panitumumab)
Anti-Human EGFR (Panitumumab)
Product No.: LT620
- -
- -
Product No.LT620 Clone ABX-EGF Target EGFR Product Type Biosimilar Recombinant Human Monoclonal Antibody Alternate Names Epidermal growth factor receptor, ErbB1, Anti-Human EGFR, ABX-EGF 339177-26-3 Isotype Human IgG2κ Applications ELISA , FA , FC , IP , WB |
Data
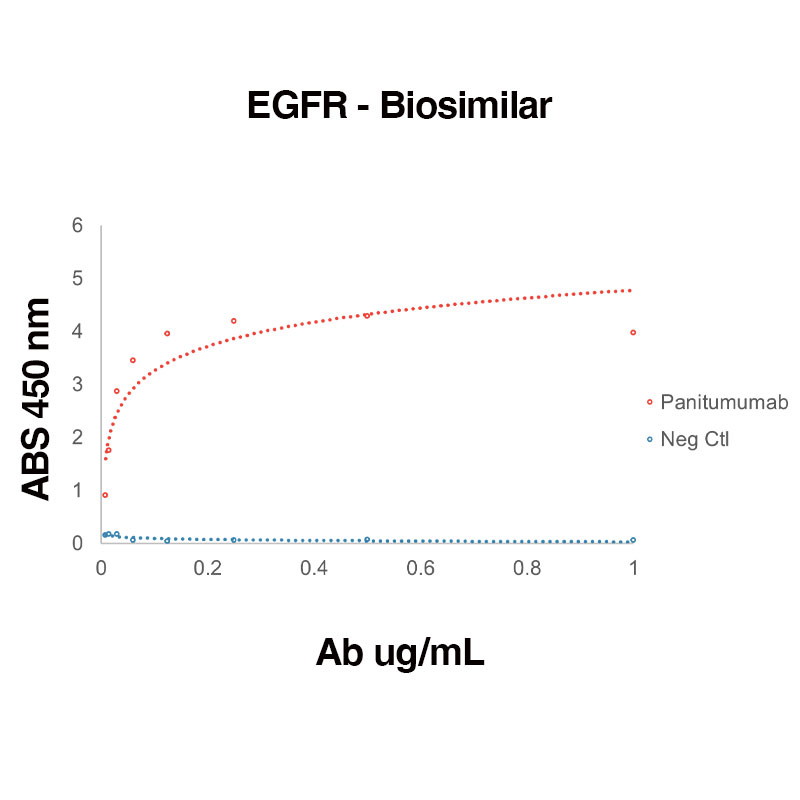 Direct binding of Human Recombinant EGFR (Leinco Prod. No.: E309) to anti-Human EGFR Panitumumab (Leinco Prod. No.: LT620)
Direct binding of Human Recombinant EGFR (Leinco Prod. No.: E309) to anti-Human EGFR Panitumumab (Leinco Prod. No.: LT620)
Binding was measured by ELISA. Recombinant Human EGFR was immobilized at 1 µg/mL. Panitumumab antibody was titrated.
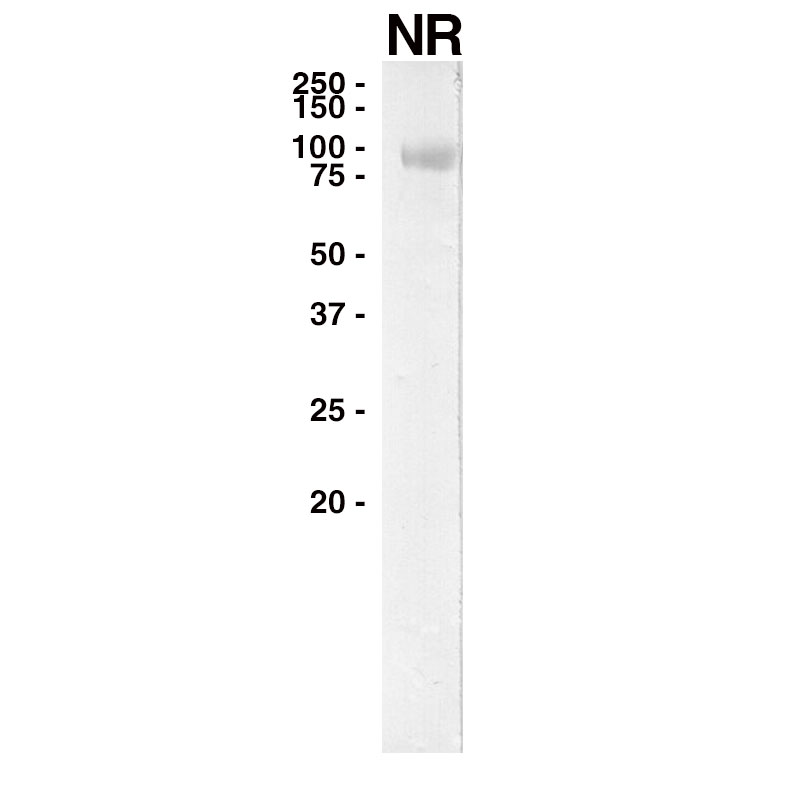 WESTERN
WESTERN
Purified recombinant human EGFR (Leinco Prod. No.: E309) was separated on SDS-PAGE under non-reducing conditions and probed with Panitumumab (Leinco Prod. No.: LT620).
- -
- -
Antibody DetailsProduct DetailsReactive Species Human Host Species Human Expression Host HEK-293 Cells FC Effector Activity Active Immunogen Human EGFR/ErbB1 Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% by SDS Page ⋅ ≥95% monomer by analytical SEC Formulation This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. State of Matter Liquid Product Preparation Recombinant biosimilar antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s recombinant biosimilar antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Regulatory Status Research Use Only (RUO). Non-Therapeutic. Country of Origin USA Shipping 2-8°C Wet Ice Applications and Recommended Usage? Quality Tested by Leinco FA, ELISA, WB Additional Applications Reported In Literature ? IP, FC Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Panitumumab. This product is for research use only. Panitumumab activity is directed against Human EGFR. Background Epidermal growth factor receptor (EGFR, also known as ErbB1 or HER-1) belongs to the receptor tyrosine kinase superfamily and is a transmembrane glycoprotein that activates various signaling pathways fundamental to cellular proliferation, differentiation, and survival1, 2. EGFR plays important roles during embryogenesis, organogenesis, and in the growth, differentiation, maintenance, and repair of adult tissues2. EGFR is also a host factor that facilitates viral entry for hepatitis B4, hepatitis C5, and gastroenteritis6 and plays a role in SARS-CoV-2 infection7, 8, 9.
Dysregulation, somatic mutation, and/or altered signaling of EGFR is associated with neurological diseases (e.g. Parkinson’s2, Alzheimer’s1, 2, and amyotrophic lateral sclerosis2) and multiple cancers (lung, glioblastoma, brain, breast, colorectal, ovarian)10. Additionally, in cancer, binding of ligands to EGFR is associated with aberrant cell proliferation, invasion, metastasis, angiogenesis, and decreased apoptosis11. As such, EGFR is the target of multiple cancer therapies, including monoclonal humanized antibodies, such as panitumumab, as well as selective small molecule inhibitors. Panitumumab was generated in a XenoMouse IgG2 strain immunized with the human cervical epidermal carcinoma cell line A43112. Panitumumab binds specifically to EGFR and inhibits the growth and survival of selected human tumor cell lines over-expressing EGFR in vitro and in vivo13. Panitumumab binds EGFR with high affinity, blocking the binding of both EGF and TGF-α, and preventing EGF-activated EGFR tyrosine autophosphorylation and downstream activation of receptor-associated kinases12. Panitumumab inhibits cell growth, tumor cell activation, in vitro tumor cell proliferation12, and metastasis13. Panitumumab also induces apoptosis and decreases proinflammatory cytokine and vascular growth factor production13. Additionally, upon binding, panitumumab causes EGFR internalization in tumor cells12. Panitumumab was approved in the United States for the treatment of some patients with EGFR-expressing metastatic colorectal cancer14, 15. Antigen Distribution EGFR is overexpressed on the cell surfaces of various tumor cell types and is also found in the plasma membranes, cytoplasm, and cell junctions of many healthy tissues, including those associated with the Skin – Epidermis development cluster of The Human Protein Atlas. EGFR is also found in the blood secretome. Ligand/Receptor Epidermal growth factor receptor PubMed NCBI Gene Bank ID UniProt.org Research Area Biosimilars . Cancer . Cell Biology . Immuno-Oncology . Immunology References & Citations1. Jayaswamy PK, Vijaykrishnaraj M, Patil P, et al. Ageing Res Rev. 83:101791. 2023.
2. Romano R, Bucci C. Cells. 9(8):1887. 2020. 3. Sigismund S, Avanzato D, Lanzetti L. Mol Oncol. 12(1):3-20. 2018. 4. Iwamoto M, Saso W, Sugiyama R, et al. Proc Natl Acad Sci U S A. 116(17):8487-8492. 2019. 5. Lupberger J, Zeisel MB, Xiao F, et al. Nat Med. 17(5):589-595. 2011. 6. Hu W, Zhang S, Shen Y, et al. Virology. 521:33-43. 2018. 7. Klann K, Bojkova D, Tascher G, et al. Mol Cell. 80(1):164-174.e4. 2020. 8. Xu G, Li Y, Zhang S, et al. Cell Res. 31(12):1230-1243. 2021. 9. Wang S, Qiu Z, Hou Y, et al. Cell Res. 31(2):126-140. 2021. 10. Sigismund S, Avanzato D, Lanzetti L. Mol Oncol. 12(1):3-20. 2018. 11. Garnock-Jones KP. Drugs. 76(2):283-289. 2016. 12. Yang XD, Jia XC, Corvalan JR, et al. Crit Rev Oncol Hematol. Apr;38(1):17-23. 2001. 13. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf 14. Dubois EA, Cohen AF. Br J Clin Pharmacol. 68(4):482-483. 2009. 15. Saltz L, Easley C, Kirkpatrick P. Nat Rev Drug Discov. 5(12):987-988. 2006. 16. Giusti RM, Shastri KA, Cohen MH, et al. Oncologist. 12(5):577-583. 2007. Technical ProtocolsCertificate of Analysis |
Formats Available
- -
- -
Prod No. | Description |
|---|---|
LT620 | |
LT625 |


