Anti-Mouse IFNγ (Clone H22) – Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse IFNγ (Clone H22) – Purified in vivo PLATINUM™ Functional Grade
Product No.: I-1190
- -
- -
Clone H22 Target IFNγ Formats AvailableView All Product Type Monoclonal Antibody Alternate Names Immune Interferon, Type II Interferon, T Cell Interferon, MAF, IFNG, IFG, IFNy Isotype IgG Applications ELISA , IF , in vivo , IP , N , WB |
Data
- -
- -
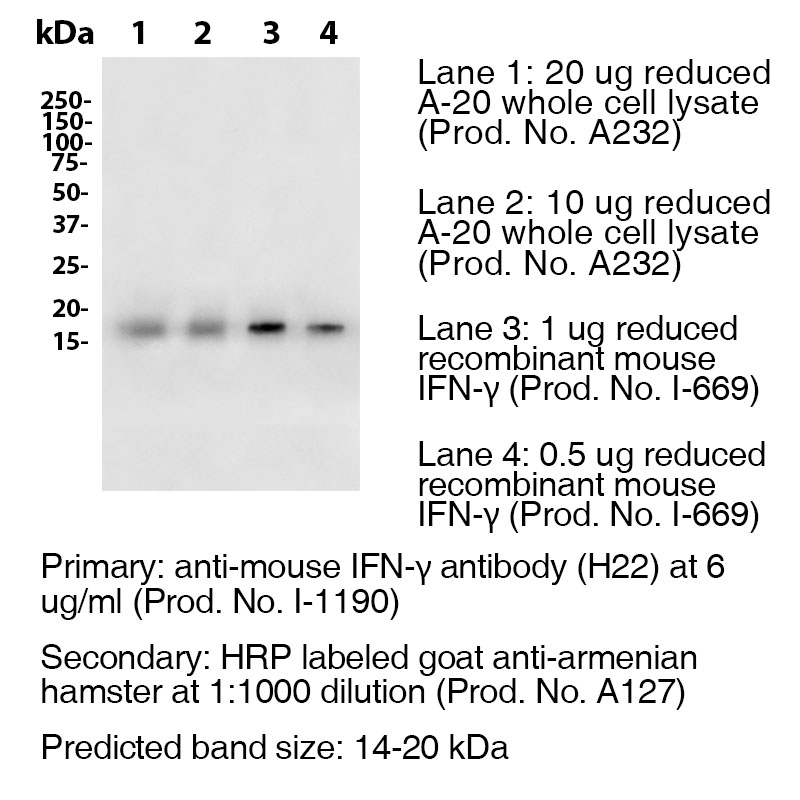
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Armenian Hamster Recommended Dilution Buffer Immunogen Purified Recombinant Mouse IFN-γ (>98%) Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2830520 Applications and Recommended Usage? Quality Tested by Leinco WB The suggested concentration for this clone H22 antibody for use in western blotting is 0.5 μg/ml. Additional Applications Reported In Literature ? N ELISA IF IP For specific conjugates of this clone, review literature for suggested application details. Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Armenian Hamster Anti-Mouse Interferon Gamma (IFN-γ) (Clone H22) recognizes an epitope on Mouse IFN-γ. This antibody was also pathogen tested and third-party certified by IDEXX BioReseach to meet the lowest mycoplasma specification and free of any viral pathogens of concern. Background Interferon-gamma (IFN-γ) or type II interferon is a dimerized soluble cytokine that is the only member of the type II class of interferons.3 It is a cytokine critical for innate and adaptive immunity against viral and intracellular bacterial infections and for tumor control. IFNG is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once antigen-specific immunity develops.4 IFN-γ has antiviral, immunoregulatory, and anti-tumour properties.5 Ligand/Receptor IFN-γRα (CDw119) dimerized with IFN-γRβ (AF-1) NCBI Gene Bank ID UniProt.org Research Area Immunology . Other Molecules References & Citations1.) Schreiber, RD. et al. (2017) Cancer Immunol Res. 5(2):106-117. PubMed 2.) Schreiber, RD. et al. (2015) PLoS One.10(5):e0128636. PubMed 3.) Goeddel, DV. et al. (1982) Nature 298: 859 4.) Wilson, CB. et al. (2007) Adv. Immunol. 96: 41 5.) Hume, DA. et al. (2004) J Leukoc Biol. 75: 163 6.) Karki et al. (2021) Cell. 184:149–168 Journal Link Technical ProtocolsCertificate of Analysis |