Anti-Mouse IFNAR-1 – Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse IFNAR-1 – Purified in vivo PLATINUM™ Functional Grade
Product No.: I-1188
- -
- -
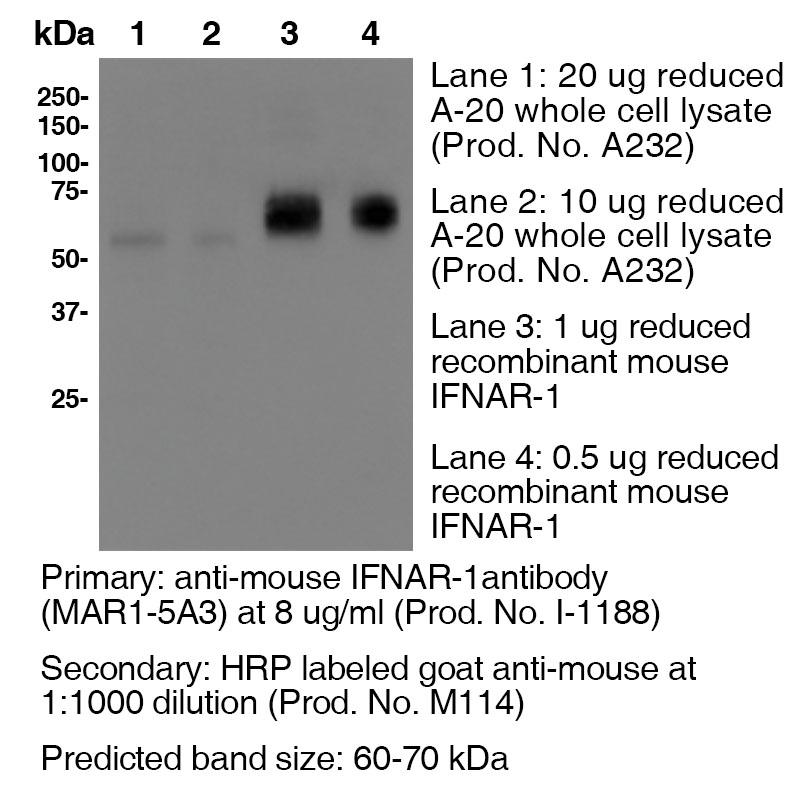
Clone MAR1-5A3 Target IFNAR1 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names CD118, Ifar, Ifnar, Ifrc, INF-a receptor, Interferon-α/β receptor α chain Isotype Mouse IgG1 Applications B , ELISA , FC , in vivo , IP , WB |
Data
- -
- -
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Mouse Recommended Isotype Controls Recommended Dilution Buffer Immunogen Plasmid DNA encoding murine IFNAR1 extracellular domain Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2830518 Applications and Recommended Usage? Quality Tested by Leinco FC The suggested concentration for clone MAR1-5A3 antibody for staining cells in flow cytometry is ≤ 2.0 μg per 106 cells in a volume of 100 μl or 100μl of whole blood. Titration of the reagent is recommended for optimal performance for each application. Additional Applications Reported In Literature ? B Clone MAR1-5A3 has a short half-life due to the rapid recycling of cells that express the IFNAR1 receptor. In order to block function In vivo, continual blocking of all compartments is necessary. Therefore, a large loading dose is necessary to saturate all In vivo binding sites and should be maintained to ensure binding site saturation. For In vivo blocking studies, a loading dose of 2.5 mg/mouse, followed by a weekly dose of 0.5 mg/mouse is recommended. The half-life following a 2.5 mg loading dose is approximately 5 days. However, if you fail to saturate the binding sites by injecting a low dose of 250 μg, for example, the half life is only 1.5 days. WB IP ELISA Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone MAR1-5A3 recognizes an epitope on mouse IFNAR1. Background IFNAR1 is a type I membrane protein, that in conjunction with IFNAR2, makes up the heterodimeric receptor that binds all type I IFNs, which includes IFN α and β. Binding and activation of the receptor stimulates Janus protein kinases, which leads to the phosphorylation of several other proteins, namely STAT1 and STAT2. IFNAR1 has also been shown to interact with PRMT1 and Tyrosine kinase 2. Type I IFNs are a family of cytokines that have been shown to promote anti-viral, anti-microbial, anti-tumor and autoimmune responses In vivo. Antigen Distribution IFNAR1 and IFNAR2 are coexpressed on nearly all cells. Ligand/Receptor IFN-α/β NCBI Gene Bank ID UniProt.org References & Citations1.) Beaver, Jacob T. et al. (2020) Human Vaccines & Immunotherapeutics 16:9, 2092-2108 Article Link 2.) Theofilopoulos, AN. et al. (2012) J Immunol. 189: 000–000. Article Link 3.) Oldstone, Michael B. A. et al. (2017) Proc Natl Acad Sci U S A. 114(14): 3708–3713. PubMed 4.) Schreiber, RD et al. (2015) PLoS One.10(5):e0128636PubMed 5.) Shin, Haina et al. (2018) J Virol. 92(7): e00038-18. PubMed 6.) Crack, Peter J. et al. (2016) eNeuro 10.1523/ENEURO.0128-15.2016 Article Link 7.) Sheehan, K. C. F. et al. (2006) JICR 26(11):804 8.) Dunn, G. P. et al. (2005) Nat. Immunol. 6(7):722 9.) Fenner, J. E. et al. (2006) Nat. Immunol. 7(1):33 10.) Biron, C. A. et al. (2007) J Exp Med. 204(10): 2383 11.) Sharma S. et al. (2020) Human Vaccines & Immunotherapeutics 16(9):2196-2203 Journal Link 12.) Cliffe, A. et al. (2021) EMBO Reports 22(9):e52547 Journal Link Technical ProtocolsCertificate of Analysis |
Formats Available
- -
- -
Prod No. | Description |
|---|---|
I-400 | |
I-1014 | |
I-1018 | |
I-402 | |
I-401 | |
I-1033 | |
I-1032 | |
I-1188 |