Anti-SARS-CoV-2 Spike RBD (Clone: 2196) – Purified No Carrier Protein
Anti-SARS-CoV-2 Spike RBD (Clone: 2196) – Purified No Carrier Protein
Product No.: LT8000
- -
- -
Product No.LT8000 Clone 2196 Target SARS-CoV-2 RBD Product Type Recombinant Monoclonal Antibody Alternate Names COV2-2196, SARS-CoV-2 Spike RBD Antibody, Receptor Binding Domain Antibody Isotype Human IgG1 Applications ELISA |
Data
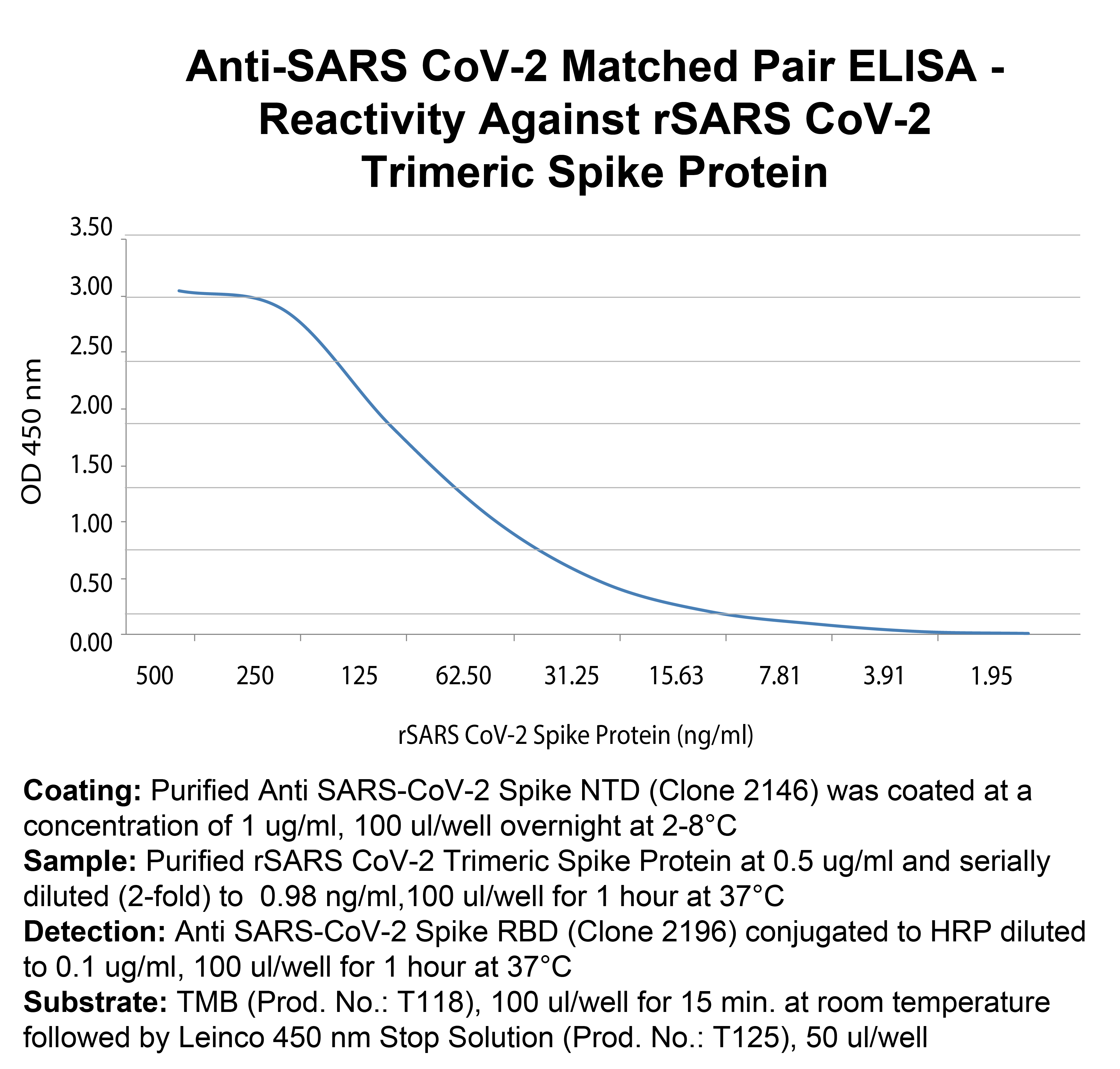 Coating: Purified Anti SARS-CoV-2 Spike NTD (Clone 2146) was coated at a concentration of 1 ug/ml, 100 ul/well overnight at 2-8°C
Sample: Purified rSARS CoV-2 Trimeric Spike Protein at 0.5 ug/ml and serially diluted (2-fold) to 0.98 ng/ml,100 ul/well for 1 hour at 37°C
Detection: Anti SARS-CoV-2 Spike RBD (Clone 2196) conjugated to HRP diluted to 0.1 ug/ml, 100 ul/well for 1 hour at 37°C
Substrate: TMB (Prod. No.: T118), 100 ul/well for 15 min. at room temperature followed by Leinco 450 nm Stop Solution (Prod. No.: T125), 50 ul/well
Coating: Purified Anti SARS-CoV-2 Spike NTD (Clone 2146) was coated at a concentration of 1 ug/ml, 100 ul/well overnight at 2-8°C
Sample: Purified rSARS CoV-2 Trimeric Spike Protein at 0.5 ug/ml and serially diluted (2-fold) to 0.98 ng/ml,100 ul/well for 1 hour at 37°C
Detection: Anti SARS-CoV-2 Spike RBD (Clone 2196) conjugated to HRP diluted to 0.1 ug/ml, 100 ul/well for 1 hour at 37°C
Substrate: TMB (Prod. No.: T118), 100 ul/well for 15 min. at room temperature followed by Leinco 450 nm Stop Solution (Prod. No.: T125), 50 ul/well - -
- -
Antibody DetailsProduct DetailsReactive Species SARS-CoV-2 ⋅ Virus Expression Host HEK-293 Cells Immunogen Sequenced from human survivors of COVID-19 (SARS-CoV-2) Product Concentration ≥1.0 mg/ml Purity ≥90% monomer by analytical SEC and SDS-Page Formulation This recombinant monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Recombinant antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling This antibody may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Standard Overnight on Blue Ice. RRIDAB_2894018 Applications and Recommended Usage? Quality Tested by Leinco ELISA Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Anti-SARS-CoV-2 Spike RBD, clone 2196, specifically targets an epitope on the SARS-CoV-2 spike protein receptor-binding domain (RBD). Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), is an enveloped, single-stranded, positive-sense RNA virus that belongs to the Coronaviridae family 1. The SARS-CoV-2 genome, which shares 79.6% identity with SARS-CoV, encodes four essential structural proteins: the spike (S), envelope (E), membrane (M), and nucleocapsid protein (N) 2. The S protein is a transmembrane, homotrimeric, class I fusion glycoprotein that mediates viral attachment, fusion, and entry into host cells 3. Each ~180 kDa monomer contains two functional subunits, S1 (~700 a.a) and S2 (~600 a.a), that mediate viral attachment and membrane fusion, respectively. S1 contains two major domains, the N-terminal (NTD) and C-terminal domains (CTD). The CTD contains the receptor-binding domain (RBD), which binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells 3-5. Although both SARS-CoV and SARS-CoV-2 bind the ACE2 receptor, the RBDs only share ~73% amino acid identity, and the SARS-CoV-2 RBD binds with a higher affinity compared to SARS-CoV 3, 6. The RBD is dynamic and undergoes hinge-like conformational changes, referred to as the “down” or “up” conformations, which hide or expose the receptor-binding motifs, respectively 7. Following receptor binding, S1 destabilizes, and TMPRSS2 cleaves S2, which undergoes a pre- to post-fusion conformation transition, allowing for membrane fusion 8, 9. Monoclonal RBD-specific antibodies can block ACE2 binding 10, 11, and anti-RBD neutralizing antibodies are present in the sera of convalescent COVID19 patients 12, identifying the RBD as an attractive candidate for vaccines and therapeutics. In addition, the RBD is poorly conserved, making it a promising antigen for diagnostic tests 13 14. Serologic tests for the RBD are highly sensitive and specific for detecting SARS-CoV-2 antibodies in COVID19 patients 13 15. Furthermore, the levels of anti-RBD antibodies correlated with SARS-CoV-2 neutralizing antibodies, suggesting the RBD could be used to predict an individual's risk of disease 13. Antigen Distribution The spike RBD is expressed on the surface of SARS-CoV-2. PubMed Research Area COVID-19 . Infectious Disease . Seasonal and Respiratory Infections . Viral . IVD Raw Material References & Citations1. Zhou, P., Yang, X., Wang, X. et al. Nature 579, 270–273. 2020. 2. Wu, F., Zhao, S., Yu, B. et al. Nature 579, 265–269. 2020. 3. Wrapp D, Wang N, Corbett KS, et al. bioRxiv. 2020.02.11.944462. 2020. 4. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Cell. 181(2):281-292.e6. 2020. 5. Li W, Zhang C, Sui J, et al. EMBO J. 24(8):1634-1643. 2005. 6. Shang, J., Ye, G., Shi, K. et al. Nature 581, 221–224. 2020. 7. Gui M, Song W, Zhou H, et al. Cell Res. 27(1):119-129. 2017. 8. Walls AC, Tortorici MA, Snijder J, et al. Proc Natl Acad Sci U S A. 114(42):11157-11162. 2017. 9. Hoffmann M, Kleine-Weber H, Schroeder S, et al. Cell. 181(2):271-280.e8. 2020. 10. Huo J, Zhao Y, Ren J, et al. Cell Host Microbe. S1931-3128(20)30351-6. 2020. 11. Tai, W., He, L., Zhang, X. et al. Cell Mol Immunol 17, 613–620. 2020. 12. Cao Y, Su B, Guo X, et al. Cell. 182(1):73-84.e16. 2020. 13. Premkumar L, Segovia-Chumbez B, Jadi R, et al. medRxiv; 2020. 14. Quinlan BD, Mou H, Zhang L, et al. bioRxiv; 2020. 15. Olba NM, Muller MA, Li W, et al. medRxiv; 2020. 16. Chen RE, Winkler ES, Case JB, et al. Nature; 2021. Technical ProtocolsCertificate of Analysis |
Formats Available
- -
- -


