Recombinant Human EGF
Data
- -
- -
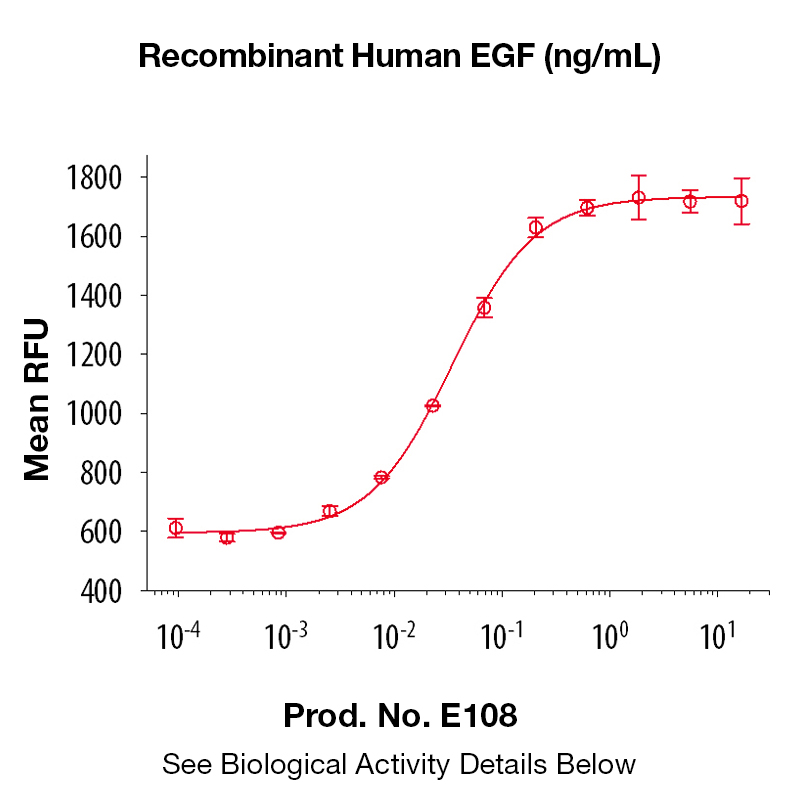
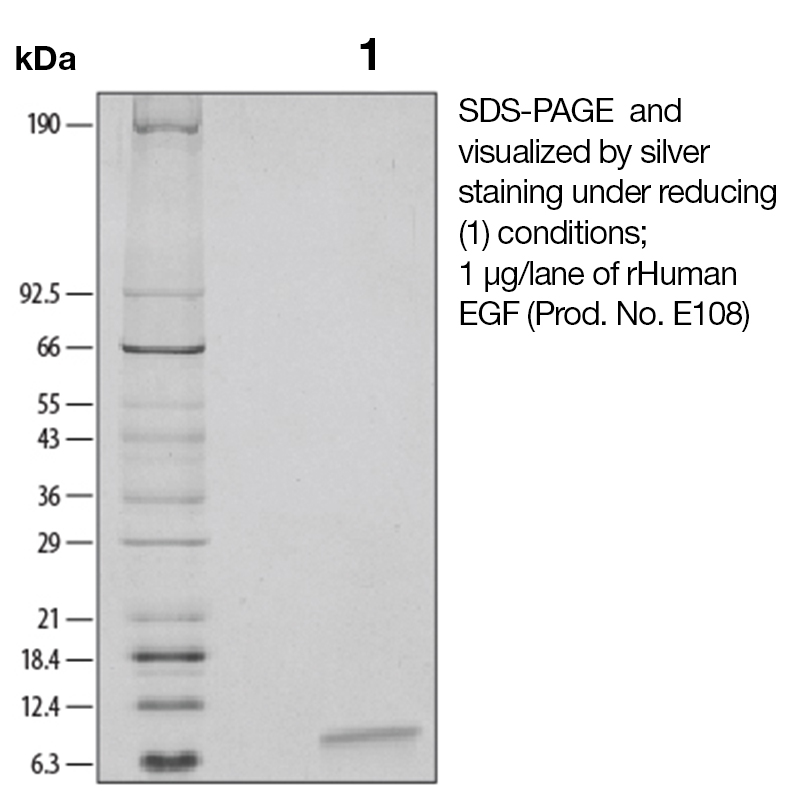
BackgroundEpidermal growth factor (EGF), also known as Urogastrone, is a growth factor that plays an important role in the regulation of cell growth, proliferation and differentiation (1). It is produced by many cell types, in blood and various body fluids, including milk, urine, saliva, seminal fluid, pancreatic juice, cerebrospinal fluid, and amniotic fluid (1). EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface, activating an extensive network of signal transduction pathways that include PI3K/AKT, RAS/ERK and JAK/STAT. It is also a mitogen for fibroblasts, epithelial and endothelial cells, and promotes colony formation of epiderma. EGF has been shown to regulate tumor cell invasion through MMP-2 activation in various tumor cell types (2). Additionally, EGF has been shown to inhibit gastric secretion, and to be involved in wound healing (3). Because of its key role in driving the proliferation of cells, EGFR is a target of several anti-cancer drugs currently in development. Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <0.1 EU/µg as determined by the LAL method Biological Activity The biological activity of Human EGF was determined by its ability to stimulate proliferation in an EGF-responsive mouse fibroblast cell line, Balb/3T3 (Rubin, J.S. et al., 1991, Proc. Natl. Acad. Sci. USA 88:415). The expected ED<sub>50</sub> is typically 20 - 100 pg/ml. Protein Accession No. Amino Acid Sequence nsdsecplsh dgyclhdgvc myiealdkya cncvvgyige rcqyrdlkww elr N-terminal Sequence Analysis Met State of Matter Lyophilized Predicted Molecular Mass The predicted molecular weight of Recombinant Human EGF is Mr 6 kDa. Additionally, the actual molecular weight as observed by migration on SDS-PAGE is 6 kDa (reducing conditions). Predicted Molecular Mass 6 Formulation This recombinant protein was 0.2 µm filtered and lyophilized from modified Dulbecco’s phosphate buffered saline (1X PBS) pH 7.2 – 7.3 with no calcium, magnesium, or preservatives. Storage and Stability This lyophilized protein is stable for six to twelve months when stored desiccated at -20°C to -70°C. After aseptic reconstitution, this protein may be stored at 2°C to 8°C for one month or at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day Ambient NCBI Gene Bank References & Citations1. Carpenter, G. et al. (1986) J. Biol. Chem. 265:770 2. Cosen-Binker, LI. et al. (2009) Biochem. Biophys. Res. Commun. 379:445 3. Machowska, A. et al. (2008) Inflammopharmacol. 16:40 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
- -
- -
Prod No. | Description |
|---|---|
E239 | |
E256 | |
E108 | |
E188 | |
E120 | |
E315 | |
E323 | |
E104 | |
E134 | |
E276 | |
E121 | |
E135 | |
B636 |