Recombinant Influenza A HA (A/California/07/2009(H1N1))
Data
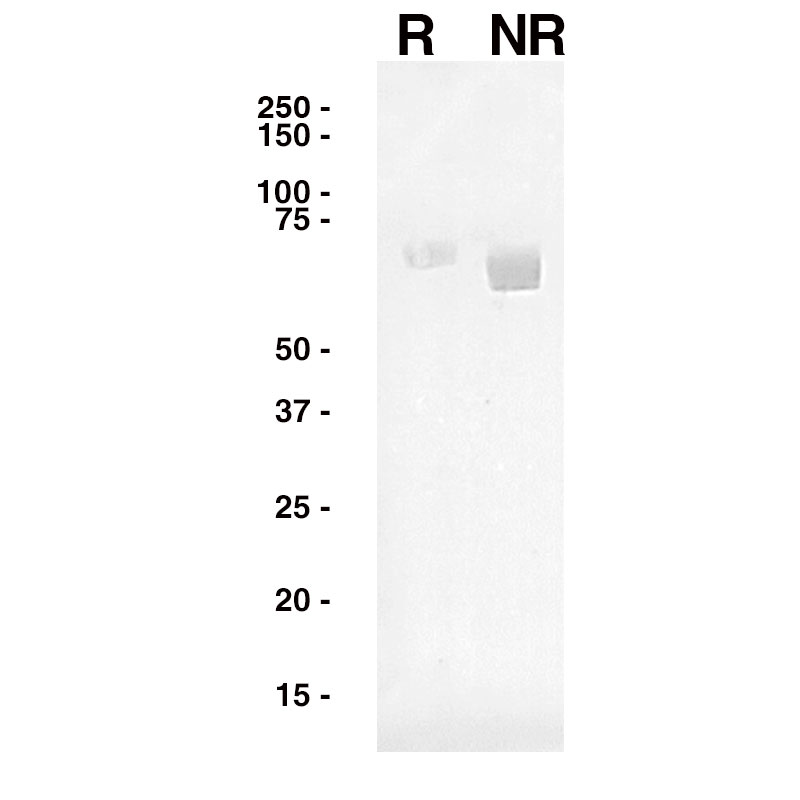 Western Blot
Western Blot
Purified Recombinant Influenza A HA (Leinco Prod. No.: F618) was separated on SDS-PAGE under both reducing and non-reducing conditions and probed with an anti-Influenza A HA antibody (Leinco Prod. No.: LT579).
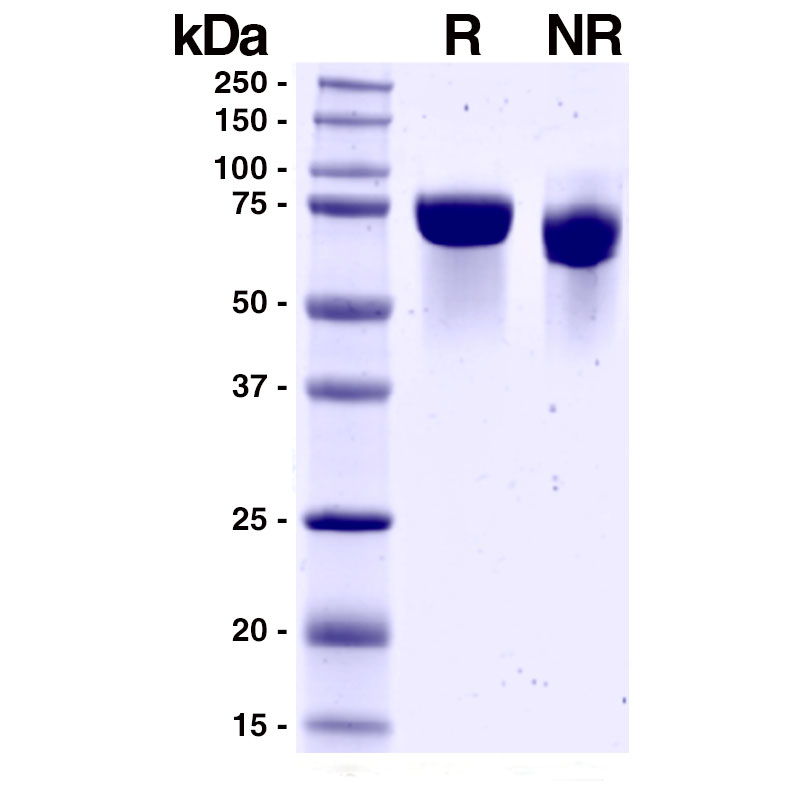 SDS-PAGE
SDS-PAGE
R: 5µg reduced Recombinant Influenza A HA (Leinco Prod. No.: F618)
NR: 5µg non-reduced Recombinant Influenza A HA (Leinco Prod. No.: F618)
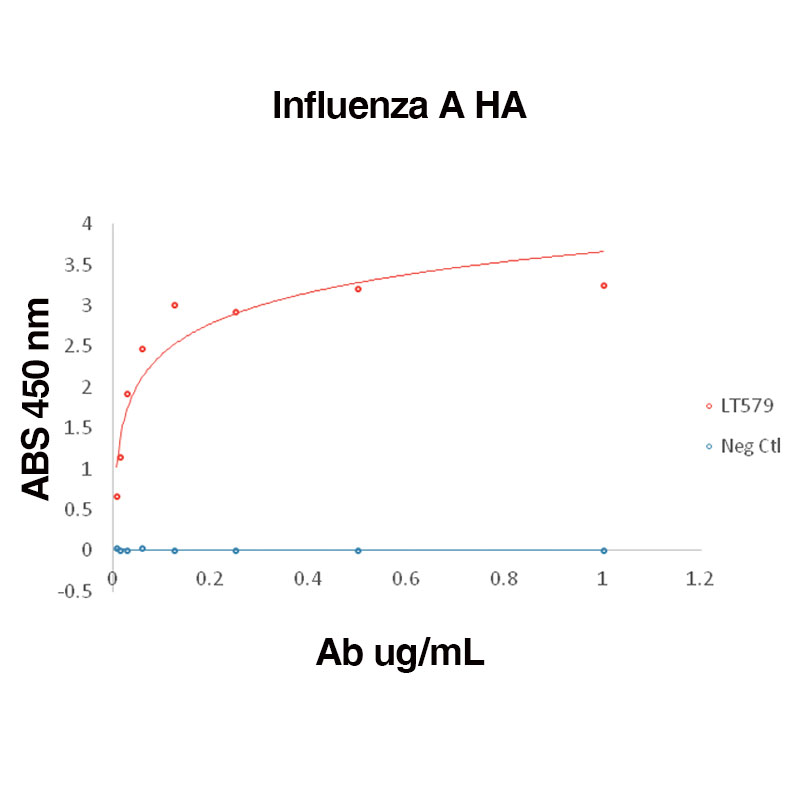 Direct binding of anti-Influenza A HA antibody (Leinco Prod. No.: LT579) to Recombinant Influenza A HA (Leinco Prod. No.: F618)
Direct binding of anti-Influenza A HA antibody (Leinco Prod. No.: LT579) to Recombinant Influenza A HA (Leinco Prod. No.: F618)
Binding was measured by ELISA. Recombinant Influenza A HA was immobilized at 1 µg/mL. Anti-Influenza A HA antibody was titrated.
- -
- -
BackgroundInfluenza viruses are members of the Orthomyxoviridae family and are phylogenetically grouped into four genera: A, B, C and D1. Influenza A viruses have two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), with HA outnumbering NA by five- to 10-fold. Based on HA antigenicity, influenza A viruses are divided into 18 known subtypes (H1-H18), and the HA subtypes are further divided into group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18) and group 2 (H3, H4, H7, H10, H14, and H15).
HA is a homotrimer consisting of a globular head domain atop a membrane-proximal stem domain1. The globular head domain of H1-H16 viruses has a membrane-distal receptor binding site that binds to sialylated glycan receptors on host cells to initiate viral entry. H17 and H18, found only in bats, instead use the major histocompatibility complex class II molecule as host receptor. HA mediates virus-host membrane fusion in a pH dependent process that occurs in the endosome. Influenza annually infects 10 to 20% of the human population causing severe illness and death2. HA is constantly evolving to escape herd immunity and vaccine preparations1. The globular head domain is immunodominant with high plasticity, while the immunosubdominant stalk domain is relatively conserved2. Aquatic birds are the primary reservoir for influenza A1. Reassortment with human and swine viruses can result in new pandemic strains. Broadly neutralizing antibodies that target relatively conserved HA domains and neutralize multiple strains are being studied1. HA can be used to rapidly quantify influenza virus using the hemagglutination assay, which takes advantage of HA’s ability to agglutinate red blood cells1. HA can also be used in the hemagglutination inhibition assay to measure the virus-neutralizing capacity of antibodies and sera. Additionally, HA is commonly used in laboratories for protein purification and labeling as an HA-tag, a linear epitope consisting of nine amino acids. Protein DetailsSpecies Viral Format Purified No Carrier Protein Purity ≥95% by SDS Page Endotoxin Level <1.0 EU/µg Biological Activity ELISA binding Protein Accession No. ACP41953.1 Amino Acid Sequence DTLCIGYHANNSTDTVDTVLEKNVTVTHSVNLLEDKHNGKLCKLRGVAPLHLGKCNIAGWILGNPECESLSTASSWSYIVETPSSDNGTCYPGDFIDYEELREQLSSVSSFERFEIFPKTSSWPNHDSNKGVTAACPHAGAKSFYKNLIWLVKKGNSYPKLSKSYINDKGKEVLVLWGIHHPSTSADQQSLYQNADAYVFVGSSRYSKKFKPEIAIRPKVRDQEGRMNYYWTLVEPGDKITFEATGNLVVPRYAFAMERNAGSGIIISDTPVHDCNTTCQTPKGAINTSLPFQNIHPITIGKCPKYVKSTKLRLATGLRNIPSIQSRGLFGAIAGFIEGGWTGMVDGWYGYHHQNEQGSGYAADLKSTQNAIDEITNKVNSVIEKMNTQFTAVGKEFNHLEKRIENLNKKVDDGFLDIWTYNAELLVLLENERTLDYHDSNVKNLYEKVRSQLKNNAKEIGNGCFEFYHKCDNTCMESVKNGTYDYPKYSEEAKLNREEIDGVKLESTRIYQ State of Matter Lyophilized SDS-Page Molecular Weight 63 kDa Predicted Molecular Mass 63 kDa Formulation Lyophilized powder from 0.01M Phosphate Buffered Saline (150mM NaCI) (PBS) pH 7.4, with 5% Trehalose Reconstitution Reconstitute at 0.1-1 mg/ml using filtered PBS. Gently mix by vortexing and/or inversion until fully dissolved. Centrifuge if necessary. Storage and Stability This lyophilized protein is stable for twelve months when stored at -20°C to -70°C. After aseptic reconstitution, this protein may be stored for one month at 2°C to 8°C or for three months at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Ambient Ligand/Receptor Ligand Species Viral Regulatory Status Research Use Only UniProt.org C3W5X2 Applications and Recommended Usage ? (Quality Tested by Leinco) SDS-PAGE, WB, ELISA References & Citations1. Wu NC, Wilson IA. Cold Spring Harb Perspect Med. 10(8):a038778. 2020.
2. Kirkpatrick E, Qiu X, Wilson PC, et al. Sci Rep. 8(1):10432. 2018. Technical ProtocolsCertificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.


