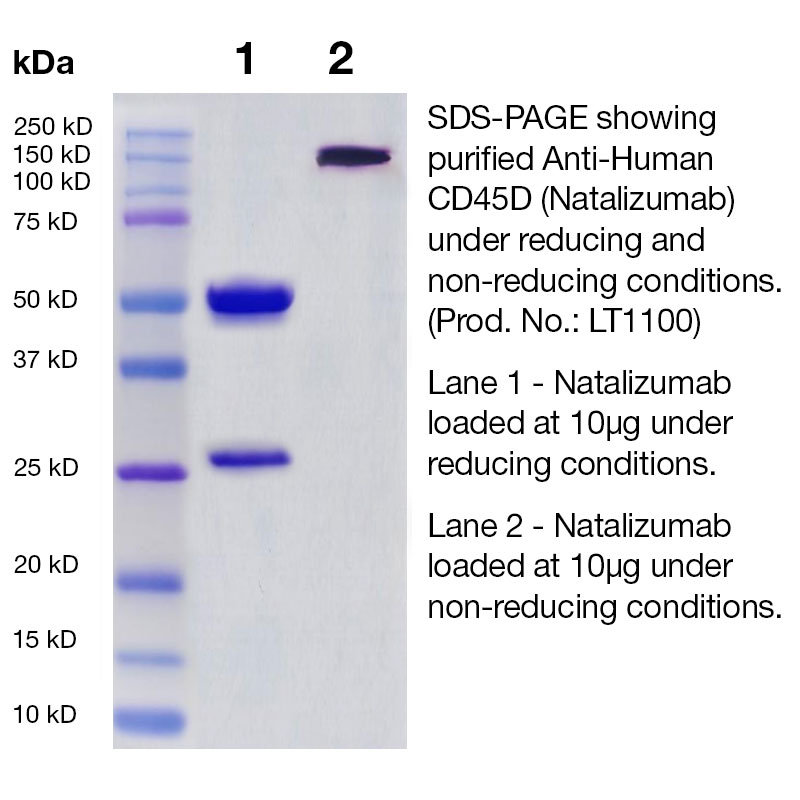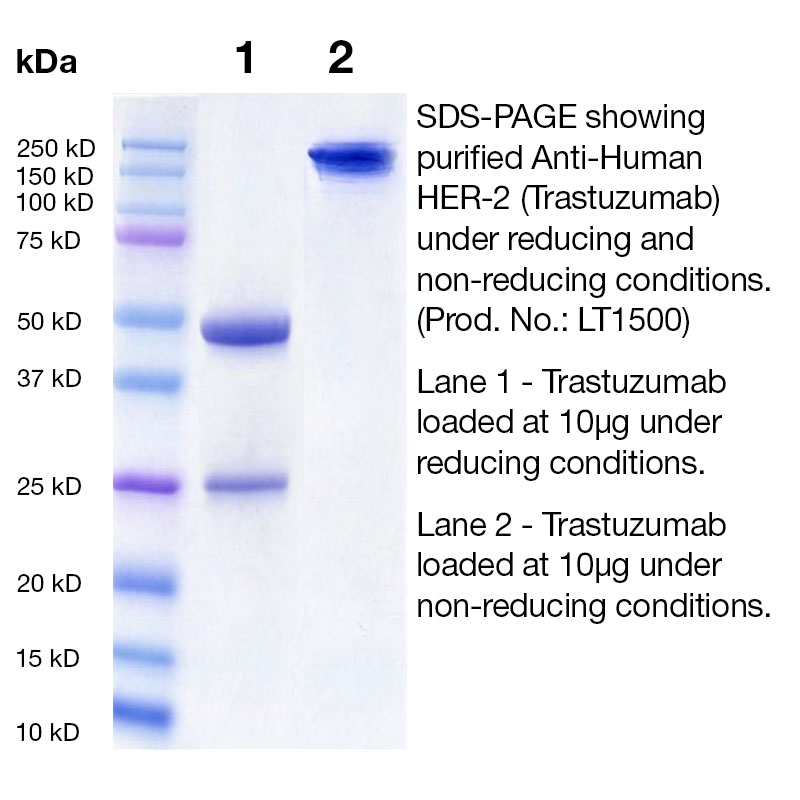
Product Specifications and IMPACT I Testing on Purified Biosimilars (RUO)
- Purity ≥ 95% by SDS-PAGE and ≥ 95% monomer by analytical SEC
- Endotoxin ≤ 1.0 EU/mg as determined by the LAL method
- Manufactured in an animal-free facility using protein-free, animal-origin-free cell culture media
- Ultra-low levels of leached protein A
- Sterile filtered and formulated in Leinco’s in vivo grade PBS pH 7.2 containing no K or Ca, additives, preservatives, or carrier proteins
- Many research applications, including FC, ELISA, IHC, FA, PK and potency assays
IDEXX IMPACT I (PCR-based) Mouse Pathogen Profile for Purified Biosimilar Abs
| Mycoplasma sp. | Mouse adenovirus 1 (MAV1) | Lactate dehydrogenase-elevating virus (LDEV) |
| Mycoplasma pulmonis | Mouse adenovirus 2 (MAV2) | Mouse kidney parvovirus (MKPV) |
| Sendai virus | Murine norovirus (MNV) | Hantaan Virus |
| Mouse hepatitis virus (MHV) | Reovirus 3 (REO3) | Mouse cytomegalovirus (mCMV) |
| Pneumonia virus of mice (PVM) | Mouse rotavirus (EDIM) | K virus |
| Minute virus of mice (MVM) | Ectromelia virus | Mouse thymic virus (MTV) |
| Mouse parvovirus (MPV) | Polyomavirus | Corynebacterium bovis |
| Theiler’s murine encephalomyelitis (TMEV) | Lymphocytic choriomeningitis virus (LCMV) | Corynebacterium sp. |